Abstract
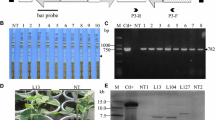
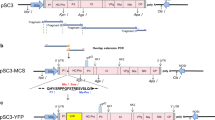
Soybean mosaic virus (SMV), a species of the Potyvirus genus in the Potyviridae family, is one of the most typical viral diseases and results in yield and quality loss of cultivated soybean. Due to the depletion of genetic resources for resistance breeding, a trial of genetic transformation to improve disease resistance has been performed by introducing the SMV-CP gene by the RNA interference (RNAi) method via Agrobacterium-mediated transformation. Among 30 transgenic plants produced, 7 lines with enough seeds were infected with SMV and two lines (3 and 4) showed viral resistance to SMV infection. In genomic Southern blot analysis, all the lines tested contained at least one T-DNA insertion. Subsequent investigation confirmed that no viral CP gene expression was detected in two SMV-resistant lines after artificial inoculation of SMV, while non-transgenic control and other transgenic lines expressed substantial amounts of the viral gene. Viral symptoms affected seed morphology, and clean seeds were harvested from the resistant lines. Also, strong viral gene expression was detected from the seeds of susceptible lines. In further generations, the same phenotypic appearance was maintained among non-transgenic and transgenic plants. Finally, the presence of helper component-proteinase (HC-Pro), known as a suppressor of gene silencing apparatus, was checked among transgenic lines. No expression of HC-Pro in resistant lines indicated that the viral CP-RNAi transformation into soybean somehow created a functional gene silencing system and resulted in a viral-resistant phenotype.





Similar content being viewed by others
References
Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB (1998) A viral suppressor of gene silencing in plants. Proc Natl Acad Sci USA 95:13079–13084
Atreya PL, Lopez-Moya JJ, Chu M, Atreya CD, Pirone TP (1995) Mutational analysis of the coat protein N-terminal amino acids involved in potyvirus transmission by aphids. J Gen Virol 76:265–270
Cho EK, Chung KW (1986) Strain of soybean mosaic virus causing soybean necrotic disease in Korea. Korean J Breed 18(2):150–153
Di R, Purcell V, Collins GB, Ghabrial SA (1996) Production of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene. Plant Cell Rep 15:746–750
Gunduz I, Buss GR, Chen P, Tolin SA (2004) Genetic and phenotypic analysis of soybean mosaic virus resistance in PI 88788 soybean. Phytopathology 94:687–692
Hannon GJ (2002) RNA interference. Nature 418:244–251
Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, Fischhoff DA, Re DB, Fraley RT, Horsch RB (1988) Production of transgenic soybean plant using Agrobacterium-mediated DNA transfer. Nat Biotechnol 6:915–922
Hobbs HA, Hartman GL, Wang Y, Hill CB, Bernard RL, Pedersen WL, Domier LL (2003) Occurrence of seed coat mottling in soybean plants inoculated with Bean pod mottle virus and Soybean mosaic virus. Plant Dis 87:1333–1336
Jagtap UB, Gurav RG, Bapat VA (2011) Role of RNA interference in plant improvement. Naturwissenschaften 98:473–492
Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J (2003) Systemic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231–237
Kang SH, Lim WS, Hwang SH, Park JW, Choi HS, Kim KH (2006) Importance of the C-terminal domain of soybean mosaic virus coat protein for subunit interactions. J Gen Virol 87:225–229
Karimi M, Inze D, Depicker A (2002) Gateway™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7(5):193–195
Kim KI, Ku JH (2006) Isolation of high quality RNA from seeds of the mungbean (Vigna radiate). Korean J Crop Sci 51(S):274–276
Lee KJ, Seo JK, Lee HY, Jeon EH, Shin SH, Lee JH, Kim DH, Ko JM, Hahn WY, Baek IY, Oh BJ, Chung YS (2006) Optimization of genetic transformation conditions for Korean soybean cultivars. Korean J Life Sci 16(2):289–296
Lim SM (1985) Resistance to soybean mosaic virus in soybeans. Phytopathology 75:199–201
Meurer CA, Dinkins RD, Collins GB (1998) Factors affecting soybean cotyledonary node transformation. Plant Cell Rep 18:180–186
Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003) Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216:723–735
Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K (2006) Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep 25:206–213
Rahman M, Ali I, Husnain T, Riazuddin S (2008) RNA interference: the story of gene silencing in plants and humans. Biotech Adv 26:202–209
Rojas MR, Zerbini FM, Allison RF, Gilbertson RL, Lucas WJ (1997) Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 237:283–295
Roth BM, Pruss GJ, Vance VB (2004) Plant viral suppressors of RNA silencing. Virus Res 102:97–108
Schwarz DS, Hutvagner G, Haley B, Zamore PD (2002) Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell 10:537–548
Seo JK, Lee SH, Kim KH (2009) Strain-specific cylindrical inclusion protein of soybean mosaic virus elicits extreme resistance and a lethal systemic hypersensitive response in two resistant cultivars. Mol Plant-Microbe Interact 22(9):1151–1159
Steinlage TA, Hill JH, Nutter FW Jr (2002) Temporal and spatial spread of soybean mosaic virus (SMV) in soybeans transformed with the coat protein gene of SMV. Phytopathology 92:478–486
Zamore PD, Tuschl T, Sharp PA, Bartel DP (2000) RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101:25–33
Zheng C, Chen P, Hymowitz T, Wickizer S, Gergerich R (2005) Evaluation of Glycine species for resistance to Bean pod mottle virus. Crop Prot 24:49–56
Zheng C, Chen P, Gergerich R (2006) Genetic analysis of resistance to soybean mosaic virus in J05 soybean. J Hered 97(5):429–437
Acknowledgments
This research was funded by Dong-A University.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. J. Kim and M.-J. Kim contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11816_2013_279_MOESM1_ESM.doc
Supplemental Fig. S1. Confirmation of SMV-CP gene insertion. DNA samples were extracted from wild-type and transgenic plants (T0) to amplify SMV-CP gene insertion in genome with PCR. a The DNA amplification between SMV-CP (forward primer) and Intron (reverse primer). b The DNA amplification between Intron (forward primer) and SMV-CP (reverse primer) (DOC 50 kb)
Rights and permissions
About this article
Cite this article
Kim, H.J., Kim, MJ., Pak, J.H. et al. Characterization of SMV resistance of soybean produced by genetic transformation of SMV-CP gene in RNAi. Plant Biotechnol Rep 7, 425–433 (2013). https://doi.org/10.1007/s11816-013-0279-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-013-0279-y