Abstract
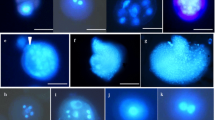
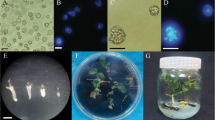
Isolated microspore culture experiments were carried out in sweet pepper (Capsicum annuum L.) F1 hybrid genotypes. In the first experiment, four culture media (W14, B5, MS and NLN) were compared to test their effectiveness in inducing the formation of microspore-derived structures in two genotypes. The experiments revealed the superiority of B5 medium. In the second experiment, the effects of different ratios of 2,4-dichlorophenoxyacetic acid (2,4-D) (0, 0.1, 0.2 and 0.5 mg l−1) and kinetin (0, 0.2 and 0.5 mg l−1) were also investigated in B5 medium with two genotypes. The effect of growth regulators were investigated on the production of microspore-derived calli and embryo-like structures (ELSs), the ratio of the two and plant regeneration (number of regenerated plantlets) in microspore culture. The histological experiments revealed the differences between the microspore-derived ELSs and calli. The most promising results were obtained on the investigated parameters in the presence of 0.1 mg l−1 2,4-D and 0.2 mg l−1 kinetin producing the highest number of plantlets in both genotypes tested. In the response of 11 genotypes, the androgenesis induction was successful in each sweet pepper genotypes tested using the best basic medium and growth regulators combination. In case of 11 genotypes, the number of ELSs ranged from 20 to 100/Petri dish (an average of 48.1 ELS/Petri dish), while the number of green plantlets varied from 0 to 8 plantlets/Petri dish (an average of 1.5 plantlets/Petri dish) depending on the genotype. The spontaneous rediploidization rate obtained was 25% in isolated microspore.



Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DH:
-
Doubled haploid
- ELS:
-
Embryo-like structure
- LSD:
-
Least significant difference
References
Csilléry G (2006) Pepper taxonomy and the botanical description of the species. Acta Agron Hung 54:151–166
Dolcet-Sanjuan R, Claveria E, Huerta A (1997) Androgenesis in Capsicum annuum L. Effect of carbohydrate and carbon dioxide enrichment. J Am Soc Hortic Sci 122:468–475
Doležel J, Binorova P, Lucretti S (1989) Analysis of nuclear-DNA content in plant-cells by flow-cytometry. Biol Plant 31(2):113–120
Dumas de Vaulx R, Chambonet D, Pochard E (1981) Culture in vitro d’anthères du piment (Capsicum annuum L.): amélior des taux d’obtention de plantes chezdifférents génotypes par des traitments ŕ+ 35 C. Agronomie 1:859–864
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gémes Juhász A, Lantos CS, Pauk J (2010) New perspective: microspore culture as new tool in paprika breeding. In: Book of advances in genetics and breeding of capsicum and eggplant XIVth EUCARPIA meeting on genetics and breeding of capsicum and eggplant, Valencia, Spain, pp 377–381
Gémesné Juhász A, Lantos C, Vági P, Kristóf Z, Pauk J (2009) In vitro anther and isolated microspore culture as tools in sweet and spice pepper breeding. Acta Hortic 829:423–431
Gémes Juhász A, Vencel G, Sági Zs, Gajdos L, Kristóf Z, Vági P, Zatykó L (2006) Production of doubled haploid breeding lines in case of paprika, eggplant, cucumber, zucchini and onion. Acta Hortic 725:845–854
George L, Narayanaswamy S (1973) Haploid Capsicum through experimental androgenesis. Protoplasma 78:467–470
Gyulai G, Gémesné Juhász A, Sági Z, Venczel G, Pintér P, Kristóf Z, Törjék O, Heszky L, Bottka S, Kiss J, Zatykó L (2000) Doubled haploid development ad PCR analysis of F-1 hybrid derived DH-R-2 paprika (Capsicum annuum L.) lines. J Plant Physiol 156:168–174
Harms CT, Potrikus I (1978) Fractionation of plant protoplast types iso-osmotic density gradient centrifugation. Theor Appl Genet 53:57–63
Heberle-Bors E (1982) In vitro pollen embryogenesis in Nicotiana tabacum L. and its relation to pollen sterility, sex balance and floral induction of the pollen donor plants. Planta 156:396–401
Horner M, Street HE (1978) Pollen dimorphism—origin and significance in pollen plant formation by anther culture. Ann Bot 42:763–771
Irikova T, Grozeva S, Rodeva V (2011) Anther culture in pepper (Capsicum annuum L.) in vitro. Acta Physiol Plant 33:1559–1570
Jedrzejczyk I, Nowaczyk P (2009) In vivo polyembryony induction in species of Capsicum. Acta Biol Cracov Bot 51:55–60
Kim M, Jang IC, Kim JA, Park EJ, Yoon M, Lee Y (2008) Embryogenenesis and plant regeneration of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep 27:425–434
Kim M, Kim J, Yoon M, Choi DI, Lee KM (2004) Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum L.). Plant Cell Tissue Org Cult 77:63–72
Kristiansen K, Andersen SB (1993) Effect of donor plant-temperature, photoperiod, and age on anther culture response of Capsicum annuum L. Euphytica 67:105–109
Kuo JS, Wang ZZ, Chien NF, Ku SJ, Kung ML, Hsu HC (1973) Investigation on the anther culture in vitro of Nicotiana tabacum L. and Capsicum annuum L. Acta Bot Sin 15:43–47
Lantos C, Gémes Juhász A, Somogyi Gy, Ötvös K, Vági P, Mihály R, Kristóf Z, Somogyi N, Pauk J (2009) Improvement of isolated microspore culture of pepper (Capsicum annuum L.) via co-culture with ovary tissues of pepper or wheat. Plant Cell Tissue Org Cult 97(3):285–293
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
Mitykó J, Andrásfalvy A, Csilléri G, Fáry M (1995) Anther-culture response in different genotypes and F1 hybrids of pepper (Capsicum annuum L.). Plant Breed 114:78–80
Mitykó J, Gémes Juhász A (2006) Improvement in the haploid technique routinely used for breeding sweet and spice pepper in Hungary. Acta Agron Hung 54:203–219
Mordhorst AP, Lörz H (1993) Embryogenesis and development of isolated barley (Hordeum vulgare L.) microspores are influenced by the amount of nitrogen sources in culture media. J Plant Physiol 142:485–492
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ochoa-Alejo N, Ramírez-Malagón R (2001) In vitro chilli pepper biotechnology. In Vitro Cell Dev Plant 37:701–729
Ouyang JW, Jia SE, Zhang C, Chen X, Fen G (1989) A new synthetic medium (W14) for wheat anther culture. In: Annual report, Institute of Genetics, Academia Sinica, Beijing, pp 91–92
Parra-Vega V, Palacios-Calvo N, Corral-Martínez P, Seguí-Simarro JM (2010) Establishment of isolated microspore cultures in pepper of the California and Lamuyo types. In: Prohens J, Rodríguez-Burruezo A (eds) Advances in genetics and breeding of Capsicum and eggplant. UPV Press, Valencia, pp 411–415
Pauk J, Lantos C, Somogyi G, Vági P, Ábrahám Táborosi Z, Gémes Juhász A, Mihály R, Kristóf Z, Somogyi N, Tímár Z (2010) Tradition. Quality and biotechnology in Hungarian spice pepper (Capsicum annuum L.) breeding. Acta Agron Hung 58:259–266
Seguí-Simarro JM, Corral-Martínez P, Parra-Vega V, González-Garcia B (2011) Androgenesis in recalcitrant solanaceous crops. Plant Cell Rep 30:765–778
Sibi M, Dumas de Vaulx R, Chambonnet D (1979) Obtention de plantes haploïdes par androgenèse in vitro chez le piment (Capsicum annuum L.). Ann Amélior Plantes 29:583–606
Simonne AH, Simonne EH, Eitenmiller RR, Mills HA, Green NR (1997) Ascorbic acid and provitamin A contents in unusually colored bell peppers (Capsicum annuum L.). Food Compo Anal 10:299–311
Steinitz B, Wolf D, Matzevitch-Josef T, Zelcer A (1999) Regeneration in vitro and genetic transformation of pepper (Capsicum spp.). The current state of art. Capsicum Eggplant Newslett 18:9–15
Steinitz B, Kusek M, Tabib Y, Paran I, Zelcer A (2003) Pepper (Capsicum annuum L.) regenerants obtained by direct somatic embryogenesis fail to develop a shoot. In Vitro Cell Dev Plant 39:296–303
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006a) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep 25:1–10
Supena EDJ, Muswita W, Suharsono S, Custers JBM (2006b) Evaluation of crucial factors for implementing shed-microspore culture of Indonesian hot pepper (Capsicum annuum L.) cultivars. Sci Hortic 107:226–232
Thomas WTB, Forster BP, Gertsson B (2003) Doubled haploids in breeding. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 95–102
Touraev A, Ilham A, Vicente O, HeberleBors E (1996) Stress-induced microspore embryogenesis in tobacco: an optimized system for molecular studies. Plant Cell Rep 15:561–565
Wang YY, Sun CS, Wang CC, Chien NF (1973) The induction of the pollen plantlets of triticale and Capsicum annuum from anther culture. Sci Sin 16:147–151
Acknowledgments
This work was supported by the Hungarian-Romanian Cross-border Co-operation Programme, 2007–2013 (HURO/0801/143, acronym: RedpepperTRD) and National Office for Research and Technology—Hungarian Scientific Research Fund (OTKA CK80719 and OTKA CK80766). The authors thank Szilvia Palaticki, Zsuzsanna Kun, Ferenc Markó for their conscientious work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lantos, C., Gémes Juhász, A., Vági, P. et al. Androgenesis induction in microspore culture of sweet pepper (Capsicum annuum L.). Plant Biotechnol Rep 6, 123–132 (2012). https://doi.org/10.1007/s11816-011-0205-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0205-0