Abstract
MuSI, a gene that corresponds to a domain that contains the rubber elongation factor (REF), is highly homologous to many stress-related proteins in plants. Since MuSI is up-regulated in the roots of plants treated with cadmium or copper, the involvement of MuSI in cadmium tolerance was investigated in this study. Escherichia coli cells overexpressing MuSI were more resistant to Cd than wild-type cells transfected with vector alone. MuSI transgenic plants were also more resistant to Cd. MuSI transgenic tobacco plants absorbed less Cd than wild-type plants. Cd translocation from roots to shoots was reduced in the transgenic plants, thereby avoiding Cd toxicity. The number of short trichomes in the leaves of wild-type tobacco plants was increased by Cd treatment, while this was unchanged in MuSI transgenic tobacco. These results suggest that MuSI transgenic tobacco plants have enhanced tolerance to Cd via reduced Cd uptake and/or increased Cd immobilization in the roots, resulting in less Cd translocation to the shoots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High biomass plants such as willows and poplars have been introduced into ecosystems for phytoextraction (Robinson et al. 2000). However, this approach is limited by the need to overcome the low heavy metal accumulation in the plants due to their low heavy metal tolerance. Transgenic plants created by genetic engineering have stronger tolerance to heavy metals or accumulate more heavy metals than non-transgenic plants (Cherian and Oliveira 2005). Some genes of interest involved in Cd tolerance have been studied. MT1 transgenic tobacco seedlings can tolerate up to 200 mM Cd (Pan et al. 1994). The roots of gshll transgenic Indian mustard grow longer and accumulate three times more total Cd per plant than wild-type plants in the presence of 0.15 mM Cd (Zhu et al. 1999). OASTL transgenic Arabidopsis can tolerate up to 300 mM Cd and produced more biomass in agar (Kawashima et al. 2004). ZntA transgenic Arabidopsis grew better than wild-type plants in the presence of 70 mM Cd (Lee et al. 2003). The genes of these transgenic plants express proteins including ones involving metallothionein and phytochelatin, which are cysteine-rich peptides that chelate heavy metals and reduce Cd toxicity by forming peptide-metal complexes (Zenk 1996; Cobbett 2000; Cherian and Oliveira 2005; Espen and D’Souza 2005).
Transcription of MuSI, a previously identified gene, is induced in response to dehydration in sweet potato roots (Ipomoea batatas L. cv. Yulmi) (Kim et al. 2009). MuSI protein is significantly homologous to putative stress-related proteins in soybean and Arabidopsis (Seo et al. 2010). Hence, the designation of MuSI is an abbreviation for multiple stress response gene I. The predicted amino acid sequence of the full-length MuSI protein is similar to that of small rubber particle proteins (SRPPs) found in rubber trees (Seo et al. 2010). MuSI expression is also induced by various stress signals including dehydration, high salt, heavy metals, oxidation, and plant hormones (Seo et al. 2010). In addition, 35S::MuSI transgenic tobacco plants exhibit markedly enhanced tolerance to heavy metal stress compared to control plants (Seo et al. 2010).
Altogether, these studies imply that MuSI may play a role in heavy metal stress tolerance. The present study sought to deduce the underlying mechanism of MuSI transgenic tobacco tolerance to Cd stress. Our results may be used to promote the use of MuSI transgenic plants in phytoremediation of Cd-contaminated soils.
Materials and methods
Construction and expression of the MuSI expression plasmid
The MuSI gene was amplified by polymerase chain reaction (PCR) from sweet potato cDNA as previously described (Seo et al. 2010) and cloned into a T&A cloning vector (Real Biotech, Taiwan). After confirming that the sequence was correct, the MuSI gene was digested with BamHI and EcoRI, and ligated into the corresponding sites of the Escherichia coli expression vector pET28a(+). E. coli cells were treated with 0.2 mM isopropyl β-d-thiogalactoside (IPTG) for 3 h at 37°C. Proteins were then extracted from the bacteria cells by sonication in cold phosphate-buffered saline (PBS) buffer containing 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μM pepstatin A, and protease inhibitor cocktail. Crude extract was collected by centrifugation (15,000g at 4°C for 30 min). Total protein concentration was determined using protein dye reagent (Bio-Rad, USA). The proteins (20 μg) were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The bands in the gels were electrophoretically transferred onto polyvinylidene fluoride membranes (Bio-Rad) for 3 h at 4°C at 180 mA. The membranes were blocked with 5% skim milk in TBST buffer containing 25 mM Tris–HCl, pH 7.4; 150 mM NaCl, and 0.05% Tween-20 for 1 h at room temperature, and incubated overnight at 4°C with an anti-His-tag antibody (1:1,000 dilution) (Millipore, Billerica, MA, USA). Blots were washed with TBST for 40 min, and incubated with an anti-mouse IgG antibody coupled to horseradish peroxidase (1:1,000 dilution) (Millipore) for 90 min at room temperature. After washing with TBST, antibody binding was visualized using ECL western blotting detection reagent (GE Healthcare, Little Chalfont, UK). Anti-DnaJ antibody (Stressgen Biotechnologies, Victoria, BC, Canada) was used as an E. coli standard control.
Recombinant protein purification
E. coli BL21 (DE3) harboring the MuSI plasmid was grown in LB medium supplemented with 100 μg/mL ampicillin with vigorous shaking (160g) at 37°C. When the culture reached the mid-log phase (A600 = 0.6), IPTG was added at a final concentration of 0.2 mM, and the culture was grown for another 4 h at 37°C. The cells were harvested by centrifugation at 5,000g for 10 min, and then washed twice with cold-PBS buffer. The resulting cell pellet was resuspended in lysis buffer (50 mM phosphate buffer, pH 8.0; 0.3 M NaCl, 10 mM imidazole, and 1 mM PMSF) and sonicated. The homogenate was centrifuged at 12,000g for 20 min at 4°C to remove cell debris. The supernatant containing soluble protein was purified by immobilized metal affinity chromatography (IMAC). The clear supernatant was loaded by gravity onto a column containing pre-equilibrated Ni–NTA affinity resin (Qiagen, Hilden, Germany). The column was washed twice with wash buffer (50 mM phosphate buffer, pH 8.0; 0.3 M NaCl, and 50 mM imidazole). The bound MuSI protein was eluted with a buffer comprised of 50 mM phosphate buffer, pH 8.0; 0.3 M NaCl, and 0.25 M imidazole.
Stress tolerance in ectopic MuSI-expressing E. coli
Mid-log phase E. coli cells (A600 = 0.6) grown at 37°C were streaked onto LB agar supplemented with 1.0 mM or 1.5 mM CdCl2 and incubated for 24–36 h at 37°C.
Plant material and Cd treatment
MuSI transgenic line 6 previously described by Seo et al. (2010) was used for this study. Transgenic T3 seeds were germinated and grown for 4 weeks on MS medium containing 50 mg/L hygromycin to select transgenic plants. Hygomycin-resistant plants were transferred to the Yamazaki solution (Yamazaki 1982) and acclimatized for 5 days in a greenhouse prior to exposure to Cd. After acclimation, the nutrient solutions in each container were replaced by nutrient solutions containing no Cd, 100 μM Cd, or 200 μM Cd. The seedlings were grown for 3 weeks; the nutrient solutions were replenished every 3 days. The solution was maintained at pH 5.8 and EC 1.2 dS m−1 during the experiment.
Scanning electron microscopy
The tobacco T3 plants leaf surface was examined using scanning electron microscopy (SEM) as previously described (Kim 2008). Pieces of the leaves were immersed overnight in modified Karnovsky’s fixative (Karnovsky 1965) consisting of 2% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.05 M sodium cacodylate buffer (pH 7.2) at 4°C and washed with the same buffer three times for 10 min each. The specimens were fixed in a second fixative solution containing 1% (w/v) osmium tetroxide in 0.05 M sodium cacodylate buffer (pH 7.2) at 4°C for 2 h. The pieces of leaf were briefly washed twice with distilled water and dehydrated in a graded ethanol series (once in 30, 50, 70, 80, and 95% ethanol for 10 min, and three times in 100% ethanol) at room temperature. The specimens were treated twice with isoamyl acetate for 10 min each, and dried in a CPD 030 critical point drier (BAL-TEC, Balzers, Liechtenstein). Tobacco leaves specimens were individually mounted on metal stubs, sputter-coated with gold, and observed by SEM (JSM-5510; JEOL, Tokyo, Japan).
Determination of relative damage content and Cd contents
After 3 weeks of Cd treatment, whole tobacco plants were harvested and separated into leaves, shoots, and roots. All plant parts were then rinsed three times with deionized water and the excess water was removed from the surface of the tissues using ash-less paper. Weight of the fresh tissues and relative damage content (RDC) were determined prior to drying in a fan-forced oven at 65°C for 3 days to perform dry weight measurement (Steyn 1959; Westerman 1990). The roots, shoots, and leaves were digested in a solution of concentrated H2SO4 and 60% HClO4 (1:10, v:v) (Cresser and Parsons 1979). The levels of Cd in the plant parts were then determined by atomic absorption spectrophotometry (AAS-6800; Shimadzu, Japan). The RDC was calculated using the following equation:
Results and discussion
In our previous study, expression of the MuSI gene isolated from sweet potato was shown to be highly induced by various stresses including dehydration, high salt, heavy metals, oxidation, and plant hormones (Seo et al. 2010). Sequence analysis showed that the full-length cDNA has a length of 998 bp with a 717-bp open reading frame and encodes 238 amino acids. The function(s) of the putative protein(s) are unknown. Stress tolerance experiments using transgenic plants over-expressing the MuSI gene showed that all independent transgenic tobacco lines (lines 1, 3, and 6) have enhanced tolerance to high temperature and drought stress (Seo et al. 2010). Based on this previous study, MuSI seems to be involved in cellular responses to Cd stress in transgenic tobacco.
Enhanced tolerance to Cd stress in transgenic E. coli
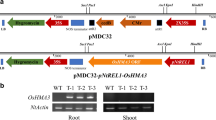
To analyze the effect of MuSI over-expression on E. coli, the MuSI gene was introduced into a E. coli expression vector (pET28a(+)::MuSI), and each construct was transformed into E. coli (Fig. 1). Ectopic over-expression of MuSI in E. coli was confirmed by immunoblotting analysis (Fig. 1). No MuSI expression was detected in cells transformed with the empty vector. After confirming MuSI over-expression, cells transformed with MuSI and the empty vector was exposed to Cd stress. Ectopic over-expression of the MuSI gene enhanced Cd tolerance of the transgenic E. coli (Fig. 1) compared to the control cells. These results suggest that MuSI may have a role in protection against Cd stress, at least in prokaryotic cells. Our findings in E. coli are similar to those from a previous study showing that MuSI is involved in defense mechanisms that act against heavy metal stress, wounding, and virus infection (Oh et al. 1999; Kim et al. 2010; Seo et al. 2010). Taken together, the results from our study and previous investigations imply that the MuSI gene may be responsible for Cd stress tolerance.
Construction of MuSI expression vector and expression in E. coli B21 cells. a Schematic diagram of the MuSI expression vector. MuSI gene with BamHI and EcoRI restriction enzyme sites was subcloned downstream to the T7 promoter (T7 P ), generating the recombinant MuSI::pET28a(+) expression vector; 6xH six histidine-tagged resides; Amp R ampicillin resistance gene. b To examine whether MuSI gene was expressed in E. coli, western blotting with anti-His tag antibody was performed. Anti-DnaJ antibody was used as a control. V Cells with the empty vector, M cells with the MuSI::pET28a(+) expression vector. c MuSI protein expression in crude protein extract and purified protein extract from E. coli cell expressing MuSI. M Protein marker, V crude protein extract from cells with the empty vector, CM crude protein extract from cells with the MuSI expression vector; PM purified MuSI protein form cells with the MuSI expression vector. d Stress tolerance of E. coli expressing MuSI in the presence of 1.0 mM (upper) or 1.5 mM (lower) CdCl2. Vector Cells with the empty vector, MuSI cells with the MuSI::pET28a(+) expression vector
Cd tolerance in transgenic plants over-expressing MuSI
Tobacco plants can absorb Cd through roots and accumulate the metal in shoots and leaves even though Cd is not an essential nutrient for plant growth and development (Welch and Norvell 1999). However, plants exposed to high concentrations of Cd show decreased growth rates and develop chlorosis (Kahel 1993; Das et al. 1997). To test whether MuSI can improve heavy metal resistance in plants, we generated transgenic tobacco plants in which MuSI expression is driven by the cauliflower mosaic virus 35S promoter (Seo et al. 2010). Four-week-old transgenic T3 and wild-type seedlings were acclimated in a hydroponic system and then treated with 100 or 200 μM CdCl2 for 3 weeks. Under these conditions, we observed a Cd resistance phenotype in the 35S::MuSI plants compared to wild-type plants (Fig. 2). Both the transgenic and wild-type plants showed visible injuries at the early stage of Cd treatment, but differences in injuries were later observed (Fig. 2a). While the wild-type plants displayed symptoms of severe toxicity at both concentrations of Cd, MuSI transgenic plants had attenuated symptoms. The leaves of the transgenic plants were in better condition than those of the wild-type plants that showed severe necrosis. Differences in plants growth rates were also noted between the transgenic and wild-type plants without Cd stress. MuSI transgenic plants showed higher growth rates than wild-type plants (Fig. 2b). However, the growth rates of both transgenic and wild-type plants were decreased in the presence of increasing Cd concentrations (Fig. 2b). Furthermore, although MuSI plants showed less damage than wild-type plants in the presence of 200 μM Cd, their growth rate was reduced as much as wild-type plants.
The tolerance of MuSI transgenic tobacco for Cd stress. a Effect of 200 μM Cd on the growth of wild-type tobacco and MuSI transgenic tobacco plants. Symptoms were visually observed at harvest. b Comparison of the fresh and dry weight of MuSI transgenic and wild-type tobacco plants treated with 0, 50, 100, or 200 μM Cd. Values are the average of six plants from each treatment. Error bars standard error. c Comparison of the growth of MuSI transgenic tobacco and wild-type tobacco plants in the presence of Cd. RDCs of the leaves from plants exposed to 50, 100, or 200 μM Cd are shown. Values are the average of six plants from each treatment. Error bars standard error. d Effect of Cd treatment on the formation of trichomes in the leaves from MuSI and wild-type tobacco plants as analyzed by SEM. Arrowheads indicate that short trichomes were sprung up by Cd treatment
RDC is the number of damaged leaves relative to the total number of total leaves and indicates the degree of leaf damage. The RDC of wild-type tobacco plants exposed to progressively higher levels of Cd increased, especially at 200 μM Cd, indicating that the seedlings were severely damaged by the Cd (Fig. 2c). In contrast, MuSI transgenic tobacco plants had a constant RDC at all tested Cd doses. Our previous study reported that three transgenic tobacco plant lines over-expressing MuSI are resistant to Cd and Cu in a germination experiment (Seo et al. 2010). The results of the present study also imply that the transgenic tobacco plants developed Cd tolerance through MuSI gene expression.
Authentic surface cell types can be distinguished by SEM. Specified portions of tobacco leaves were used for SEM analysis. The leaf cells were characterized by the presence of globular-tipped trichomes, stomata, and irregularly shaped cells. The trichomes of MuSI transgenic plants were altered in terms of size irregularities and increased density (Fig. 2d). Tobacco trichomes have head cells coated with polysaccharides containing terpenoid (Choi et al. 2004) which transform into large crystals as Cd accumulates (Salt et al. 1995; Choi et al. 2001). This resistance mechanism involves the formation of complexes containing Cd thiol compounds and translocated metallothioneins and phytochelatins, and subsequent accumulation of these complexes in vacuoles, trichomes, leaves, and shoots (Choi et al. 2001; Song et al. 2003; Kawashima et al. 2004).
The SEM observations made in the present were consistent with this mechanistic scenario. Compared to Cd-free control plants, the number of short trichomes in the leaves (0.4 mm × 0.25 mm) doubled in plants treated with 200 μM Cd. On the other hand, the number of short trichomes was not increased by Cd treatment (Fig. 2d) in the head cells of trichomes in MuSI transgenic tobacco plants that harbored modified (larger) crystals. These results suggest that the resistance mechanism of MuSI transgenic tobacco plants involves reduced Cd translocation from the roots and leaves. Put another way, the mechanism underlying the recovery of transgenic tobacco plants grown in a high concentrations of Cd is mainly due to impaired Cd uptake rather than sequestration of Cd. This is achieved by the formation of complexes containing thiol compounds in thricomes and/or Cd immobilization around the roots, resulting in decreased Cd translocation to the shoots. Longer exposure of MuSI transgenic tobacco plants to Cd could be used to more accurately measure Cd accumulation.
Cd contents of transgenic MuSI tobacco plants
Wild-type tobacco plants showed increased accumulation of Cd and suffered from more damage in a Cd concentration-dependent manner (Fig. 3). In particular, chlorosis and reduced growth rates were observed in wild-type plants. In contrast, MuSI transgenic tobacco plants avoided Cd toxicity by blocking Cd absorption in the roots (Figs. 2, 3). In the present study, MuSI transgenic tobacco plants accumulated less Cd than wild-type plants. When grown in the presence of 200 μM Cd, wild-type plants were found to contain 4.65 ± 0.32 mg of Cd per plant while and transgenic plants contained 2.37 ± 0.12 mg per plant. There was also a difference in the amount of Cd transported from the roots to leaves. In 200 μM Cd, the percent of Cd translocated to the leaves of wild-type plants was 81.6% while that of the MuSI transgenic plants was 37.1% (Fig. 3). MuSI expression may repress Cd translocation from roots to shoots, leading to an overall decrease of Cd concentration in the leaves. These data suggest that MuSI may be playing unknown important role(s) in regulating Cd absorption in roots and translocation to the shoots.
Several genes such as hMTll, YCF1, and cysteine synthase, confer resistance to Cd toxicity in transgenic tobacco or Arabidopsis plants (Maiti et al. 1989; Elmayan and Tepfer 1994; Song et al. 2003; Kawashima et al. 2004). In previous studies, tobacco seedlings expressing a MT gene encoding the metal chelating protein metallothionein showed a modified distribution of Cd in various plant parts (Maiti et al. 1989; Elmayan and Tepfer 1994). Elmayan and Tepfer (1994) reported that Cd accumulation in the leaves of transgenic seedling was reduced and Cd translocation rate of transgenic plants (20%) to the shoots also decreased comparing with that of control plants (50%). These results are similar to the ones from the present study, and indicate that Cd resistance is closely related to regulating Cd distribution in the plant. Our findings demonstrate that Cd tolerance of MuSI transgenic tobacco plants may be associated with decreased Cd absorption and transport to shoots compared to wild-type plants.
In conclusion, the results from our study show that MuSI gene expression confers tolerance to Cd stress. MuSI transgenic tobacco plants display less Cd-mediated damage than wild-type tobacco plants when grown in the presence of Cd. This observation seems to be related to decreased translocation of Cd from the roots to the leaves in the MuSI transgenic plant compared to their wild-type counterparts. Although MuSI transgenic plants did not accumulate Cd in their shoots, they might still be useful for phytoremediation processes, such as phytostabilization, in which plants reduce the mobility and bioavailability of contaminants in soil and water by immobilization (Cherian and Oliveira 2005). Thus, future studies should focus on Cd detoxification mechanisms in MuSI transgenic plants, such as ones involving metallothionein and phytochelatin, as well as the suitability of MuSI transgenic plants for phytoremediation of Cd-contaminated soil.
References
Cherian S, Oliveira MM (2005) Transgenic plants in phytoremediation: recent advance and new possibilities. Environ Sci Technol 39(24):9377–9390
Choi YE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H (2001) Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 213:45–50
Choi YE, Harada E, Kim GH, Yoon ES, Sano H (2004) Distribution of elements on tobacco trichomes and leaves under cadmium and sodium stresses. Plant Biol 47(2):75–82
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Cresser MS, Parsons JW (1979) Sulfuric-perchloric acid digestion of plant material for the determination of nitrogen, phosphorus, potassium, calcium and magnesium. Anal Chem Acta 109:431–436
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Poll 98:29–36
Elmayan T, Tepfer M (1994) Synthesis of a bifunctional metallothionein/β-glucuronidase fusion protein in transgenic tobacco plants as a means of reducing leaf cadmium levels. Plant J 6:433–440
Espen S, D’Souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23:97–114
Kahel H (1993) Response of roots of trees to heavy metals. Environ Exp Bot 33:99–119
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Kawashima CG, Noji M, Nakamura M, Ogra Y, Suzuki KT, Saito K (2004) Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol Lett 26:153–157
Kim KW (2008) Visualization of micromorphology of leaf epicuticular waxes of the rubber tree Ficus elastic by electron microscopy. Micron 39:976–984
Kim SH, Song WK, Kim YH, Kwon SY, Lee HS, Lee IC, Kwak SS (2009) Characterization of full-length enriched expressed sequence tags of dehydration-treated white fibrous roots of sweetpotato. J Biochem Mol Biol 42(5):271–276
Kim EY, Seo YS, Lee H, Kim WT (2010) Constitutive expression of CaSRP1, a hot pepper small rubber particle protein homolog, resulted in fast growth and improved drought tolerance in transgenic Arabidopsis plants. Planta 232:71–83
Lee JH, Bae HJ, Jeong JY, Lee JY, Yang YY, Hwang IH, Martinoia E, Lee YS (2003) Functional expression of a bacterial heavy metal transporter in Arabidopsis enhances resistance to and decreases uptake of heavy metals. Plant Physiol 133:589–596
Maiti IB, Wagner GJ, Yeargan R, Hunt AG (1989) Inheritance and expression of the mouse metallothionein gene in tobacco. Plant Physiol 91:1020–1024
Oh SK, Kang HS, Shin DH, Yang JM, Chow KS, Yeang HY, Wagner B, Breiteneder H, Han KH (1999) Isolation, characterization, and functional analysis of a novel cDNA clone encoding a small rubber particle protein from Hevea brasiliensis. J Biol Chem 274:17132–17138
Pan A, Tie F, Duau Z, Yang M, Wang Z, Li L, Chen Z, Ru B (1994) α-Domain of human metallothionein IA can bind to metals in transgenic tobacco plants. Mol Gen Genet 242:666–674
Robinson BH, Mills TM, Petit D, Fung LE, Green SR, Clothier BE (2000) Natural and induced cadmium-accumulation in poplar and willow: implications for phytoremediation. Plant Soil 227:301–306
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Seo SG, Kim JS, Yang YS, Jun BK, Kang SW, Lee GP, Kim W, Kim JB, Lee HU, Kim SH (2010) Cloning and characterization of the new multiple stress responsible gene I (MuSI) from sweet potato. Genes Genom 32:552–554
Song WY, Sohn EJ, Martinoia E, Lee YJ, Yang YY, Jasinski M, Forestier C, Hwang I, Lee YS (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 21:914–919
Steyn WJA (1959) Leaf analysis. Errors involved in the preparative phase. J Agric Food Chem 7:344–348
Welch RM, Norvell WA (1999) Mechanisms of cadmium uptake, translocation and deposition in plants. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plant. Kluwer, Dordrecht, pp 125–150
Westerman RL (1990) Soil testing and plant analysis, 3rd edn. SSSA book series, no. 3, pp 389–427
Yamazaki K (1982) Nutrient solution culture (Japanese). Pak-kyo, Tokyo, p 251
Zenk MH (1996) Heavy metal detoxification in higher plants. Gene 179:21–30
Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–79
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kim, YN., Kim, JS., Seo, SG. et al. Cadmium resistance in tobacco plants expressing the MuSI gene. Plant Biotechnol Rep 5, 323–329 (2011). https://doi.org/10.1007/s11816-011-0186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0186-z