Abstract
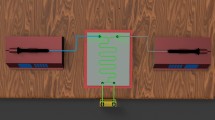
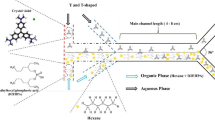
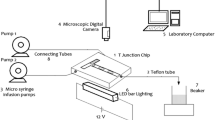
We investigated the performance of a T-type microchannel for mercaptan extraction from light straight-run naphtha (LSRN) with sodium hydroxide solution. The aim of this work is to introduce the microfluidic system as a potential tool for mercaptan extraction from light petroleum products. Modeling the extraction process of mercaptan from LSRN has not been carried out previously. In this regard, mercaptan extraction was modeled by response surface methodology (RSM) and artificial neural network (ANN) to analyze the effect of operating parameters on the mercaptan extraction process. The independent variables are considered as temperature, sodium hydroxide concentration, and the volume ratio of sodium hydroxide to LSRN. Two models were compared based on error analysis of the predicted data. Root mean square error, mean relative error, and determination coefficient for the neural network were 0.5650, 0.4341, and 0.9862, respectively. The values of these parameters for the RSM model were 0.6854, 0.7648, and 0.9798. The results showed that the prediction accuracy for both models is appropriate, but the precision of the neural network model is slightly higher than that of the RSM model. The genetic algorithm (GA) technique determined the optimal values of the independent variables with the aim of maximizing the extraction percentage. The mercaptan extraction percentage value of 85.08% was achieved at 303.15 K, the sodium hydroxide concentration of 20 wt%, and the volume ratio of sodium hydroxide to LSRN of 0.128. Furthermore, results showed a higher mercaptan extraction percentage of the microfluidic system compared to a conventional extractor at the same process condition.
Similar content being viewed by others
Abbreviations
- b:
-
bias
- COS:
-
carbonyl sulfide
- CS2 :
-
carbon disulfide
- E:
-
extraction percentage
- e:
-
error term
- F:
-
transfer function
- K E :
-
overall extraction constant
- M:
-
mercaptan concentration [ppm]
- m:
-
number of input variables
- N:
-
number of data points
- n:
-
number of neurons
- R2 :
-
coefficient of determination
- RS− :
-
ionized mercaptan
- RSH:
-
mercaptan
- W:
-
weight
- X:
-
independent variables
- Y:
-
predicted response of RSM model
- y:
-
predicted response of ANN model
- a:
-
average
- aq:
-
aqueous phase
- H:
-
hidden layer
- in:
-
inlet
- max:
-
maximum
- min:
-
minimum
- norm:
-
normalized
- org:
-
organic phase
- out:
-
outlet
- t:
-
target data
- ANN:
-
artificial neural network
- ANOVA:
-
analysis of variance
- CCD:
-
central composite design
- GA:
-
genetic algorithm
- KORC:
-
kermanshah oil refinery company
- LPG:
-
liquefied petroleum gas
- LSRN:
-
light straight-run naphtha
- MRE:
-
mean relative error
- RMSE:
-
root mean square error
- RSM:
-
response surface methodology
References
M. Khalkhali, A. Ghorbani and B. Bayati, Polyhedron, 171, 403 (2019).
D. Yabroff, Ind. Eng. Chem., 32, 257 (1940).
B. Basu, S. Satapathy and A. Bhatnagar, Catal. Rev. Sci. Eng., 35, 571 (1993).
R. Barzamini, C. Falamaki and R. Mahmoudi, Fuel, 130, 46 (2014).
Q. Liu, M. Ke, P. Yu, F. Liu, H. Hu and C. Li, Korean J. Chem. Eng., 35, 137 (2018).
M. R. Ehsani, A. R. Safadoost, R. Avazzadeh and A. Barkhordari, Iran. J. Chem. Chem. Eng., 32, 71 (2013).
C. J. LaFoy, US Patent, 4,705,620 (1985).
R. Liu, D. Xia, Y. Xiang and Y. Tian, Pet. Sci. Technol., 23, 711 (2005).
A. S. Afshar, S. R. Hashemi, M. Miri and P. Setayeshi, Pet. Sci. Technol., 31, 2364 (2013).
S. Ganguly, N. Rathi and A. Jain, Pet. Sci. Technol., 31, 1283 (2013).
M. Shahrak, E. Ebrahimzadeh and F. Shahraki, Energy Sources Part A, 37, 791 (2015).
P. Amani, M. Amani and R. Hasanvandian, Korean J. Chem. Eng., 34, 1456 (2017).
A. Parvareh, Iran. J. Chem. Eng., 14, 55 (2017).
A. Akopyan, B. Andreev, A. Anisimov, E. Eseva, A. Tarakanova, A. Ustinov, A. Kleimenov, D. Kondrashev, D. Khrapov and R. Esipenko, Russ. J. Appl. Chem., 92, 865 (2019).
Y. S. Huh, S. J. Jeon, E. Z. Lee, H. S. Park and W. H. Hong, Korean J. Chem. Eng., 28, 633 (2011).
K.-I. Sotowa, R. Miyoshi, C.-G. Lee, Y. Kang and K. Kusakabe, Korean J. Chem. Eng., 22, 552 (2005).
M. N. Kashid, A. Gupta, A. Renken and L. Kiwi-Minsker, Chem. Eng. J., 158, 233 (2010).
K. K. Singh, A. U. Renjith and K. T. Shenoy, Chem. Eng. Process., 98, 95 (2015).
L. Zhang, F. Xie, S. Li, S. Yin, J. Peng and S. Ju, Green Process. Synth., 4, 3 (2015).
S. Dai, J. Luo, J. Li, X. Zhu, Y. Cao and S. Komarneni, Ind. Eng. Chem. Res., 56, 12717 (2017).
M. Al-Azzawi, F. S. Mjalli, A. Al-Hashmi, T. Al-Wahaibi and B. Abu-jdayil, Chem. Eng. Process., 140, 43 (2019).
X. Chen, T. Li and Z. Hu, Microsyst. Technol., 23, 2649 (2017).
X. Chen and J. Shen, Int. J. Heat. Mass. Transfer., 106, 593 (2017).
M. Darekar, N. Sen, K. Singh, S. Mukhopadhyay, K. Shenoy and S. Ghosh, Hydrometallurgy, 144, 54 (2014).
A. Talebi, T. T. Teng, A. F. Alkarkhi, I. Norli and L. W. Low, Desalination. Water. Treat., 47, 334 (2012).
M. Asadollahzadeh, H. Tavakoli, M. Torab-Mostaedi, G. Hosseini and A. Hemmati, Talanta, 123, 25 (2014).
M. Karmakar, M. Mahapatra and N. R. Singha, Korean J. Chem. Eng., 34, 1416 (2017).
S. Yildiz, Korean J. Chem. Eng., 34, 2423 (2017).
B. Kim, Y. Choi, J. Choi, Y. Shin and S. Lee, Korean J. Chem. Eng., 37, 1 (2020).
S. Uslu, Fuel, 276, 117990 (2020).
UOP163-10, Hydrogen Sulfide and Mercaptan Sulfur in Liquid Hydrocarbons by Potentiometric Titration, ASTM International, West Conshohocken, PA, 2010, www.astm.org.
A. S. Afshar and S. R. Hashemi, Int. J. Chem. Biomol. Eng., 79, (2011).
M. Filiz, N. Sayar and A. Sayar, Hydrometallurgy, 81, 167 (2006).
G. R. Daham, A. A. AbdulRazak, A. S. Hamadi and A. A. Mohammed, Korean J. Chem. Eng., 34, 2435 (2017).
A. Fazlali, P. Koranian, R. Beigzadeh and M. Rahimi, Korean J. Chem. Eng., 30, 1681 (2013).
M. Izadi, M. Rahimi and R. Beigzadeh, Chem. Eng. J., 356, 570 (2019).
M. Rahimi, M. Hajialyani, R. Beigzadeh and A. A. Alsairafi, Chem. Eng. Res. Des., 98, 147 (2015).
H. Sahraie, M. R. Mirani, M. H. Ahmadi and M. Ashouri, Energy Convers. Manage., 99, 81 (2015).
M. R. Mirani and F. Rahimpour, J. Chromatogr. A, 1422, 170 (2015).
Acknowledgements
The authors wish to express thanks to the Kermanshah Oil Refinery Company (KORC) for preparing the LSRN samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirani, M.R., Fazlali, A. & Rahimi, M. Experimental and modeling studies for intensification of mercaptans extraction from LSRN using a microfluidic system. Korean J. Chem. Eng. 38, 1023–1031 (2021). https://doi.org/10.1007/s11814-021-0749-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0749-9