Abstract
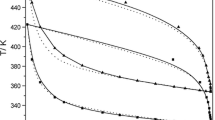
The paper reports the solid-liquid phase equilibria (SLE), excess molar volume (VE), and molar refraction deviation (ΔR) for binary systems of ethanoic acid with the C3 to C5 carboxylic acids, propanoic, butanoic, and pentanoic acid, which are the main constituents of bio-butanol fermentation broth. The SLE was determined via a synthetic method using a custom-built glass tube at atmospheric pressure, whereas the thermodynamic mixture properties, VE and ΔR were obtained from directly measured density and refractive index using a precision densitometer and refractometer, respectively. All of the SLE that were determined for binary mixtures of ethanoic acid+C3-C5 carboxylic acids showed a single eutectic point and regressed well with the NRTL activity model within 0.6 K of RMSD. The VE values for the same binaries were positive for the entire composition ranges of all the systems, whereas the ΔR values were negative for all the systems. The VE and ΔR were well regressed by polynomial equations, namely Redlich-Kister within 0.006 cm3·mol−1 of the standard deviation for VE and 0.02 cm3·mol−1 for ΔR.
Similar content being viewed by others
References
P. H. Pfromm, V. Amanor-Boadu, R. Nelson, P. Vadlani and R. Madl, Biomass Bioenergy, 34, 515 (2010).
C. Hergueta, M. Bogarra, A. Tsolakis, K. Essa and J. M. Herreros, Fuel, 208, 662 (2017).
Y. S. Jang, A. Malaviya, C. H. Cho, J. M. Lee and S.Y. Lee, Bioresour. Technol., 123, 653 (2012).
K. Karimi, M. Tabatabaei, I. S. Horvath and R. Kumar, Biofuel Res. J., 301 (2015).
S. Xie, C. Yi and X. Qiu, J. Chem. Eng. Data, 58, 3297 (2013).
D. Rabari and T. Banerjee, Fluid Phase Equilib., 355, 26 (2013).
S. Zheng, H. Cheng, L. Chen and Z. Qi, J. Chem. Thermodynam., 93, 127 (2016).
G. H. Lee and S. J. Park, Fluid Phase Equilib., 436, 47 (2017).
I. Malijevska, Fluid Phase Equilib., 211, 257 (2003).
M. Tadie, I. Bahadur, P. Reddy, P.T. Ngema, P. Naidoo, N. Deenadayalu and D. Ramjugernath, J. Chem. Thermodynam., 57, 485 (2013).
K. Granados, J. Gracia-Fadrique, A. Amigo and R. Bravo, J. Chem. Eng. Data, 51, 1356 (2006).
I. Bahadur, S. Singh, N. Deenadayalu, P. Naidoo and D. Ramjugernath, Thermochi. Acta, 590, 151 (2014).
H. Renon and J. M. Prausnitz, AIChE J., 14, 135 (1968).
D. Abrams and J.M. Prausnitz, AIChE J., 21, 116 (1975).
O. Redlich and A.T. Kister, Ind. Eng. Chem., 40, 345 (1948).
S.H. Shin, I. H. Jeong, Y. S. Jeong and S. J. Park, Fluid Phase Equilib., 376, 105 (2014).
S.H. You, I. H. Jeong, I.C. Hwang and S. J. Park, Fluid Phase Equilib., 383, 21 (2014).
S. J. Park, R. H. Kwon and Y.Y. Choi, Fluid Phase Equilib., 361, 130 (2014).
I.Y. Jeong, R. H. Kwon, S. J. Park and Y.Y. Choi, J. Chem. Eng. Data, 59, 289 (2014).
A. Jakob, R. Joh, C. Rose and J. Gmehling, Fluid Phase Equilib., 113, 117 (1995).
T.M. Aminabhavi and B. Gopalakrishna, J. Chem. Eng. Data, 40, 856 (1995).
H. Akaike, IEEE Trans. Automat. Contr., AC-19, 716 (1974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, KH., Gu, JE., Oh, HY. et al. Solid-liquid phase equilibria, excess molar volume, and molar refraction deviation for the mixtures of ethanoic acid with propanoic, butanoic, and pentanoic acid. Korean J. Chem. Eng. 35, 1710–1715 (2018). https://doi.org/10.1007/s11814-018-0072-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-018-0072-2