Abstract
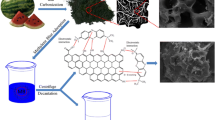
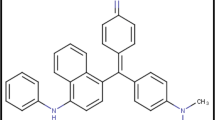
A new type of activated carbon, torreya-grandis-skin-based activated carbon (TAC), has been used to remove the harmful dyes (cationic dye crystal violet (CV) and anionic dye naphthol green (NG)) from contaminated water via batch adsorption. The effects of solution pH, adsorption time and temperature were studied. The Langmuir and Freundlich adsorption models were used to describe the equilibrium isotherm and isotherm constant calculation. It was found that the maximum equilibrium adsorption capacities were 292mg/g and 545mg/g for CV and NG, respectively. Adsorption kinetics was verified by pseudo-first-order, pseudo-second-order and intra-particle diffusion kinetic models. Results indicated that the rate of dye adsorption followed pseudo-second-order kinetic model for the initial dye concentration range studied. Temperature-dependent adsorption behavior of CV and NG shows that the adsorption is spontaneous and endothermic, accompanying an entropy increase. This work indicates that TAC could be employed as a low-cost alternative for the removal of the textile dyes from effluents.
Similar content being viewed by others
References
G. Crini, Bioresour. Technol., 97, 1061 (2006).
B.H. Hameed and A. A. Rahman, J. Hazard. Mater., 160, 576 (2008).
P. Vandevivere, R. Bianchi and W. Verstraete, J. Chem. Technol. Biotechnol., 72, 289 (1998).
Y. Slokar and A. L. Marechal, Dyes Pigm., 37, 335 (1998).
S. Wang, Z. H. Zhu, A. Coomes, F. Haghseresht and G.Q. Lu, J. Colloid Interface Sci., 284, 440 (2005).
D. Prahas, Y. Kartika, N. Indraswati and S. Ismadji, Chem. Eng. J., 140, 32 (2008).
M. P. Elizalde-González, J. Mattusch and R. Wennrich, Bioresour. Technol., 99, 5134 (2008).
R. Zhu, Q. Chen, H. Liu, F. Ge, L. Zhu, J. Zhua and H. He, Appl. Clay Sci., 88–89, 33 (2014).
I.D. Mall, V.C. Srivastava and N.K. Agarwal, Dyes Pigm., 69, 210 (2006).
A. E. Ofomaja, Chem. Eng. J., 143, 85 (2008).
B. H. Hameed, J. Hazard. Mater., 154, 204 (2008).
Y. Cao, H. Zhu and X. Wang, J. Zhejiang Normal Univ. China, 31, 190 (2008).
L. Wang, X. Chen, Y. Dong and H. Lu, Acta Agriculturae Zhejiangensis, 22, 229 (2010).
X. Sun, W. Dai, H. Lv, Y. Du, R. Gong and J. Hu. China Patent, CN103288083A (2013).
L. Ai, C. Zhang and L. Meng, J. Chem. Eng. Data, 56, 4217 (2011).
B. H. Hameed, A.T. M. Din and A. L. Ahmad, J. Hazard. Mater., 141, 819 (2007).
J. Yi and L. Zhang, Bioresour. Technol., 99, 2182 (2008).
I. Langmuir, J. Am. Chem. Soc., 38, 2221 (1916).
H. M. F. Freundlich, Z. Phys. Chem., 57, 385 (1906).
S. Langergen and B.K. Svenska, Veteruskapsakad Handlingar, 24, 1 (1898).
Y. S. Ho and G. Mckay, Process Biochem., 34, 451 (1999).
G. Annadurai, R. S. Juang and D. J. Lee, J. Hazard. Mater., 92, 263 (2002).
S. Chen, J. Zhang, C. Zhang, Q. Yue, Y. Li and C. Li, Desalination, 252, 149 (2010).
L. C. Juang, C. C. Wang and C. K. Lee, Chemosphere, 64, 1920 (2006).
S. Senthilkumaar, P. Kalaamani and C.V. Subburaam, J. Hazard. Mater., 136, 800 (2006).
C. Akmil-basar, J. Hazard. Mater., 135, 232 (2006).
R. Malarvizhi and Y. S. Ho, Desalination, 264, 97 (2011).
D. Tolga, R.K. Ali, O. Yunus, D. Erkan, A. Salih and Z.F. Turkmenoglu, Physicochem. Probl. Miner. Process., 48, 253 (2012).
M. F. Attallah, I. M. Ahmed and M. M. Hamed, Environ. Sci. Pollut. Res. Int., 20, 1106 (2013).
B.M. Elzbieta and D. Adamczyk, Rocznik ochrona srodowiska, 15, 966 (2013).
F. Zhang, Z. Ni, S. Xia, X. Liu and Q. Wang, Chinese J. Chem., 27, 1767 (2009).
K. G. Bhattacharyya and A. Sharma, Dyes Pigm., 65, 51 (2005).
J. Wei, R. Zhu, J. Zhu, F. Ge, P. Yuan, H. He and C. Ming, J. Hazard. Mater., 166, 195 (2009).
K. G. Bhattacharyya and A. Sharma, J. Hazard. Mater., 113, 97 (2004).
K. Mahmoudi, N. Hamdi, A. Kriaa and E. Srasra, Russian J. Phys. Chem. A., 86, 1294 (2012).
N. E.Q. Emad, S. J. Allen and G. M. Walker, Chem. Eng. J., 124, 103 (2006).
S. Karagoz, T. Tay, S. Ucar and M. Erdem, Bioresour. Technol., 99, 6214 (2008).
Z. Belala, M. Jeguirim, M. Belhachemi, F. Addoun and G. Trouvé, Desalination, 271, 80 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, W., Yu, H., Ma, N. et al. Adsorption equilibrium and kinetic studies of crystal violet and naphthol green on torreya-grandis-skin-based activated carbon. Korean J. Chem. Eng. 32, 335–341 (2015). https://doi.org/10.1007/s11814-014-0219-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0219-8