Abstract
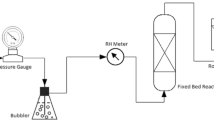
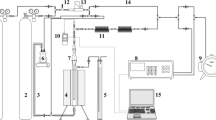
The viability of two solid adsorbents, limestone and cement powder, for use in a flow-through packed-bed column for HCl and HF gas neutralization following refrigerant destruction was studied. Neutralization tests performed at 408 K using 5% HCl in N2 and 5% HF in N2, showed that limestone had a significantly higher adsorption capacity for both HF and HCl. ∼49% of fed HCl, and between 7.8%–16.2% of fed HF gases were adsorbed by 7 g of limestone for a gas flow rate of 6.67×10−6 m3/s (STP) over 30 to 180 minutes. Effective diffusivities (D e ) of HCl and HF into the limestone particles were 1.5×10−9 and 2.2×10−9 m2/s, respectively, indicating that a solid diffusion mechanism dominance would limit the suitability of this method as a solid adsorbent in the tested form. Under these conditions, complete particle conversion times were 227 hours for HCl-limestone and 154 hours for HF-limestone. Considering the long conversion times observed, shorter conversion times would require micron-scale particle sizes, suitable for entrained flow but not for a packed-bed arrangement. A Na2CO3/Limestone slurry used to neutralize the reactor effluent proved efficient within this system, and may be a more suitable alternative for acid neutralization involving HF.
Similar content being viewed by others
References
O. Badr, S. D. Probert and P.W. O’Callaghan, Appl. Energy, 37(4), 247 (1990).
J. C. Dickerman, Technologies for CFC (Chlorofluorocarbons)/halon destruction, Radian Corp.: Research Triangle Park, NC, USA (1989).
J. Heberlein, Thermal plasmas for the destruction of hazardous wastes, in Conference proceedings — Italian Physical Society, Editrice Compositori: Bologna, 18 (1994).
J. Heberlein and A. B. Murphy, J. Phys. D: Appl. Phys., 41(5), 053001 (2008).
T. Watanabe and S. Shimbara, Halogenated Hydrocarbon Decomposition by Steam Thermal Plasmas, ChemInform, 36(25) (2005).
S. Yasui, T. Shojo, G. Inoue, K. Koike, A. Takeuchi and Y. Iwasa, Int. J. Chem. Eng., 2012, 1 (2012).
P. R. Jena, S. De and J. K. Basu, Chem. Eng. J., 95(1–3), 143 (2003).
S. Homma, S. Ogata, J. Koga and S. Matsumoto, Chem. Eng. Sci., 60(18), 4971 (2005).
K. Lee and O. Koon, Chem. Eng. J., 146(1), 57 (2009).
H. Y. Sohn and J. Szekely, Chem. Eng. Sci., 27(4), 763 (1972).
V. L. Hartmann, Chem. Eng. J., 134(1–3), 190 (2007).
S. Yagi and D. Kunii, Symposium (International) on Combustion, 5(1), 231 (1955).
O. Levenspiel, Chemical reaction engineering, New York, Wiley (1999).
C. Y. Wen, Ind. Eng. Chem., 60(9), 34 (1968).
H. S. Fogler, Elements of chemical reaction engineering, Upper Saddle River, NJ, Prentice Hall PTR (2006).
B. E. Poling, J. M. Prausnitz and J. P. O’Connell, The properties of gases and liquids, New York, McGraw-Hill (2001).
C. R. Alpass, J. Murphya, A. Jainb and P. R. Wilshaw, J. Electrochem. Soc., 156(8), H669 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akuetteh, T., Donaldson, A.A. Limestone’s performance as a solid adsorbent for HF and HCl generated in refrigerant destruction applications. Korean J. Chem. Eng. 31, 1885–1891 (2014). https://doi.org/10.1007/s11814-014-0150-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0150-z