Abstract
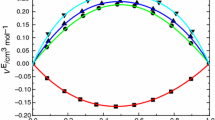
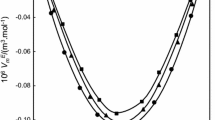
Experimental values of the density and viscosity have been measured for binary mixtures of N-ethylaniline with isomeric butanols (1-butanol, 2-butanol, 2-methyl-1-propanol and 2-methyl-2-propanol) at 303.15, 308.15 and 313.15 K over the entire mole fraction range. These data, the excess molar volumes, and deviation viscosity for the binary systems at the above-mentioned temperatures were calculated and fitted to Redlich-Kister equation to determine the fitting parameters and the root-mean-square deviations. The excess molar volumes, deviation viscosity and excess Gibbs energy of activation of viscous flow have been analyzed in terms of acid-base interactions, hydrogen bond, and dipole-dipole interaction between unlike molecules. The results obtained for dynamic viscosity of binary mixtures were used to test the semi-empirical relations of Grunberg-Nissan, Katti-Chaudhri, and Hind et al. equations.
Similar content being viewed by others
References
M. Gowrisankar, P. Venkateswarlu, K. Siva Kumar and S. Sivarambabu, J. Mol. Liq., 173, 172 (2012).
M. Gowrisankar, P. Venkateswarlu, K. Siva Kumar and S. Sivarambabu, J. Soln., Chem., 42(5), 916 (2013).
M. Gowrisankar, P. Venkateswarlu, K. Siva Kumar and S. Sivarambabu, Korean J. Chem. Eng., 30(5), 1131 (2013).
M. Gowrisankar, P. Venkateswarlu, K. Siva Kumar and S. Sivarambabu, J. Ind. Eng. Chem., DOI:10.1016/j.jiec.2013.04.035 (2013).
M. Gowrisankar, P. Venkateswarlu, K. Siva Kumar and S. Sivarambabu, Arabian J. Chem., DOI:10.1016/j.arabjc 2013.09.042 (2013).
J. K. Das, S. K. Dash, N. Swain, B. B. Swain, J. Mol. Liq., 81, 163 (1999).
Mahendra Nath Roy, Anuradha Sinha and Biswajit Sinha, J. Soln. Chem., 34, 1311 (2005).
P. S. Nikam, N. Laxman Shirsat and Mehdi Hasan, J. Chem. Eng. Data, 43, 732 (1998).
B. L. Shama, J. B. Madhuri, K. L. Machhindra and R. A. Balasaheb, J. Chem. Eng. Data, 54, 127 (2008).
W.-L. Weng, J. Chem. Eng. Data, 44, 63 (1998).
S.-d. Chen, Q.-f. Lei and W.-j. Fang, Fluid Phase Equilib., 234, 22 (2005).
J. A. Riddick, W. Bunger and T. K. Sakano, fourth Ed., Wiley Interscience, New York (1986).
Timmermans, J. Physico-chemical constants of pure organic compounds, Elsevier, Amsterdam (1950).
A. K. Nain, Fluid Phase Equilib., 259, 218 (2007).
R. Palepu, J. Diver and D. Campell, J. Chem. Eng. Data, 30, 355 (1985).
A. K. Nain, Int. J. Thermophys., 28, 1228 (2007).
S. L. Oswal, K. D. Prajapti, P. Oswal, N.Y. Ghael and S. P. Ijardar, J. Mol. Liq., 116, 73 (2005).
I.A. Mrityunjaya and G. B. Jagadish, J. Chem. Thermodyns., 38, 434 (2006).
S. T. Jyostna and N. Satyanarayana, Indian J. Chem., 44A, 1365 (2005).
B. Ranjith Kumar, B. Sathyanarayana, S.A. Banu, K. A. Jyothi, T. S. Jyostna and N. Sathyanarayana, Indian J. Pure Appl. Phys., 47, 511 (2009).
B. Ranjith Kumar, P. Murali Krishna, B. Sathyanarayana, T. S. Jyostna and N. Sathyanarayana, Indian J. Pure Appl. Phys., 47A, 1026 (2008).
R. J. Fort and W. R. Moore, Trans Faraday Soc., 62, 1112 (1966).
L. Pikkarainan, J. Chem. Eng. Data, 28, 344 (1983).
T. M. Reed and T. E. Taylor, J. Phys. Chem., 63, 58 (1959).
E.V. Vedernikova, M. M. Gafurov and M. B. Ataev, Russ. Phys. J., 53, 843 (2011).
O. Redlich and A. T. Kister, Ind. Eng. Chem., 40, 345 (1948).
Q. Lei and Y. Hou, Fluid Phase Equilib., 154, 153 (1999).
Q. Lei, Y. Hou and R. Lin, Fluid Phase Equilib., 140, 221 (1997).
L. Grunberg and A. H. Nissan, Nature, 164, 799 (1949).
P. K. Katti and M. H. Chaudhri, J. Chem. Eng. Data, 9, 442 (1964).
R. K. Hind, E. McLaughlin and A. Ubbelohde, Trans Faraday Soc., 56, 328 (1960).
M. Tamura and M. Kurata, Bull., Chem., Soc., Japan, 25, 32 (1952).
R. K. Nigam and B. S. Mahl, Indian J. Chem., 9, 1255 (1971).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaik, J., Sankar, M.G., Ramachandran, D. et al. Orientation effect on sign and magnitude of excess thermodynamic functions of non electrolyte solutions at different temperatures (303.15 K, 308.15 K, and 313.15K). Korean J. Chem. Eng. 31, 1460–1469 (2014). https://doi.org/10.1007/s11814-014-0088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0088-1