Abstract
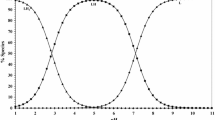
Solution equilibria of the binary and ternary complex systems of the divalent transition metal ions Cu2+, Ni2+, Zn2+, and Co2+ with 1,2,4-triazole (TRZ), 3-mercapto-1,2,4-triazole (TRZSH), and 3-amino-1,2,4-triazole (TRZAM) and aromatic carboxylic acids (phthalic, anthranilic, salicylic, and 5-sulfosalicylic acid) have been studied pH-metrically at (25.0±0.1) °C, and a constant ionic strength I=1×10−1 mol L−1 NaNO3 in an aqueous medium. The potentiometric titration curves show that binary and ternary complexes of these ligands are formed in solution. The stability constants of the different binary and ternary complexes formed were calculated on the basis of computer analysis of the titration data. The relative stability of the different ternary complex species is expressed in terms of Δ log K values, log X and R. S.% parameters. The effect of temperature of the medium on both the proton-ligand equilibria for TRZAM and phthalic acid and their metal-ligand equilibria with Cu2+, Ni2+, and Co2+ has been studied along with the corresponding thermodynamic parameters. The complexation behavior of ternary complexes is ascertained using conductivity measurements. In addition, the formation of ternary complexes in solution has been confirmed by using UV-visible spectrophotometry.
Similar content being viewed by others
References
M. Kidwai, B. Dave, P. Misra, R. K. Saxena and M. Singh, Inorg. Chem. Commun., 3, 465 (2000).
M. Kidwai, P. Sapra, P. Misra, R. K. Saxena and M. Singh, Bioorganic Medicinal. Chem., 9, 217 (2001).
J. C. Garcia-Glez, R. Mendez and J. Martin-Villacorta, J. Chromatogr., 812, 213 (1998).
I. Küçükgüzel, S. Güniz Küçükgüzel, S. Rollas and M. Kiraz, Bioorganic Medicinal. Chem., 11(13), 1703 (2001).
S. Moreau, P. Coudert, C. Rubat, D. Vallee-Goyet, G. Dardette, J. C. Gramain and J. Couquelet, Bioorganic Medicinal. Chem., 6(7), 983 (1998).
S. Manfredini, C. B. Vicentini, M. Manfrini, N. Bianchi, C. Rutigliano, C. Mischiati and R. Gambari, Bioorganic Medicinal. Chem. Commun., 8(9), 2343 (2000).
A. R. Katritzky, C.W. Rees and T. P. Kevin, Comprehensive heterocyclic chemistry: the structure, reactions, synthesis, and uses of heterocyclic compounds. Five-membered rings with two or more nitrogen atoms, Pergamon Press, Oxford, U.K., 785 (1984).
P. K. Kadaba, J. Med. Chem., 31, 196 (1988).
H. L. Hoffman, E. J. Ernst and M. E. Klepser, Expert Opin. Invest. Drugs, 9, 593 (2000).
K. Nomiya, K. Tsuda and N. C. Kasuga, J. Chem. Soc., Dalton Trans., 1653 (1998).
F. P. Dwyer and D. P. Mellor, Chelating agents and metal chelates, Academic Press, New York (1964).
A. García-Raso, J. J. Fiol, B. Adrover, P. Tauler, A Pons, I. Mata, E. Espinosa and E. Molins, Polyhedron, 22, 3255 (2003).
J. D. Ranford and P. J. Sadler, Dalton Trans., 3393 (1993).
G. Majella, S. Vivienne, M. Malachy, D. Michael and M. Vickie, Polyhedron, 18, 2931 (1999).
D.K. Saha, U. Sandbhor, K. Shirisha, S. Padhye, D. Deobagkar, C.E. Ansond and A. K. Powell, Med. Chem. Lett., 14, 3027 (2004).
M. A. Zoroddu, S. Zanetti, R. Pogni and R. Basosi, J. Inorg. Biochem., 63, 291 (1996).
H. Sigel, Metal ions in biological systems, Marcel Dekker, New York, 3 (1974).
M. Aljahadi, A. A. El-sherif, M.M. Shoukry and S. E. Mohamed, J. Solu. Chem., 42(5), 1028 (2013).
M. Baraldi, W. Malavasi and R. Grandi, J. Chem. Crystallogr., 26, 63 (1996).
D. B. Wang, B. H. Chen and Y. Xiang, Synthesis and Reactivity in Inorg., Met. Org.; Nano-Met. Chemistry, 27, 479 (1997).
N. Saravanan and K. K. M. Yusuff, Transition Met. Chem., 21, 464 (1996).
M. Gabryszewski, Spectrosc. Lett., 34, 57 (2001).
S. Goel, O. P. Pandey and S. K. Sengupta, Thermochim. Acta, 133, 359 (1988).
M. Gabryszewski, Pol. J. Chem., 68, 1895 (1994).
B. Barszcz, Pol. J. Chem., 63, 9 (1989).
M. Gabryszewski, Pol. J. Chem., 66, 1067 (1992).
B. Lenarcik, K. Kurdziel and M. Gabryszewski, J. Inorg. Nucl. Chemistry, 42, 587 (1980).
M. A. Neelakantan, M. Sundaram and N. M. Sivasankaran, J. Spectrochim. Acta Part A, 79, 1693 (2011).
S. Panda, R. Mishra, A. K. Panda and K. C. Satpathy, J. Indian Chem. Soc., 66, 472 (1989).
M. S. Reddy, K. Ram and M.G. R. Reddy, Indian J. Chem., 28A, 437 (1989).
M.M. Khalil and A. E. Fazary, Monatschefte fur Chemie, 135, 1455 (2004).
M.M. Khalil, M.M. El-Deeb and R. K. Mahmoud, J. Chem. Eng. Data, 52, 1571 (2007).
M.M. Khalil and R. K. Mahmoud, J. Chem. Eng. Data, 53, 2318 (2008).
M.M. Khalil, A.M. Radalla and A.G. Mohamed, J. Chem. Eng. Data, 54, 3261 (2009).
G. Grans, Analyst, 77, 661 (1952).
F. J. Welcher, The analytical uses of ethylenediaminetetraacetic acid, Von Nostrand: Princeton, NJ (1965).
P. Grans, B. O’Sullivan, Talanta, 51, 33 (2000).
H.M. Irving and H. S. Rossotti, J. Chem. Soc., 3397 (1953).
H.M. Irving and H. S. Rossotti, J. Chem. Soc., 2904 (1954).
P. Gans and A. Vacca, Talanta, 21, 45 (1974).
R. M. Smith and A. E. Martell, NIST critically selected stability constants of metal complexes database, Version 3.0; NIST standard reference database 46; U.S. Department of Commerce. National Institute of Standard and Technology (1997).
R.C. Weast, Handbook of chemistry and physics, CRC Press, Boca Raton, FL (1973).
A. A. A. Boraei and N. F. A. Mohamed, J. Chem. Eng. Data, 47, 987 (2002).
J. Catalan, M. Menendez and J. Elguero, Bull. Soc. Chim. Fr., 30 (1985).
J. Inezedy, Determination of equilibrium constants in: Analytical applications of complex equilibria, Ellis Horwood, Chichester, UK (1976).
K. Irving and R. P. Williams, Nature (London), 162, 746 (1948).
K. A. Idriss, M.M. Seleim, E. El-shahawy, M. B. Saleh and H. Sedaria, Monatsh. Chem., 119, 683 (1988).
G. Venkatnarayana, S. J. Swamy and P. Lingaiah, Indian J. Chem., 27A, 613 (1988).
M. Mohamed, A. I. Said, M. A. Ali and T.A. Iman, Transition Met. Chem., 21, 1 (1996).
R. B. Martin and R. J. Prados, J. Inorg. Chem., 36, 1665 (1974).
H. Sigel, Chem. Int. Edn., 14, 394 (1975).
R. Dewitt and J. L. Watters, J. Am. Chem. Soc., 76, 3810 (1954).
S. Kida, Bull. Chem. Soc. Jpn., 29, 805 (1956).
N. Sanaie and C. A. Hayres, J. Chem. Eng. Data, 50, 1848 (2005).
O. E. Offiong, Transition. Met. Chem., 23, 553 (1998).
B. N. Figgs, Introduction to ligand fields, Interscience Publishers, New York (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalil, M.M., Radalla, AE., Qasem, F. et al. Equilibrium studies of ternary systems containing some selected transition metal ions, triazoles and aromatic carboxylic acids. Korean J. Chem. Eng. 31, 109–119 (2014). https://doi.org/10.1007/s11814-013-0181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0181-x