Abstract
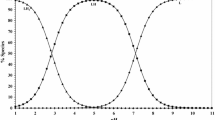
Proton–ligand association constants of imidazole-4-acetic acid (IMA) were determined potentiometrically in aqueous solution at different temperatures in the range 15–35 °C. The stepwise stability constants of IMA with some selected bivalent transition metal ions were also determined in 0.1 mol·dm−3 NaNO3. The stability of the complexes follows the trend Cu2+ > Ni2+ > Co2+ > Mn2+, which is in agreement with the Irving–Williams order of the metal ions. The thermodynamic parameters for Cu(II)–IMA complex formation were derived and discussed. The ternary complexes Cu(IMA)L (IMA = imidazole-4-acetic acid, HL = amino acid, amides or DNA constituents) have been investigated. Ternary complexes of amino acids or amides are formed by a simultaneous mechanism. Amino acids form the complex Cu(IMA)L, whereas amides form two complex species Cu(IMA)L and Cu(IMA)(LH−1). The DNA constituents form both 1:1 and 1:2 complexes. The stabilities of ternary complexes are quantitatively compared with their corresponding binary complexes. The concentration distribution of the complexes in solution was evaluated as a function of pH.











Similar content being viewed by others
Abbreviations
- IMA:
-
Imidazole-4-acetic acid hydrochloride
- GLuA:
-
Glutamine
- Ino:
-
Inosine
- Gly:
-
Glycine
- Pyr:
-
Pyrocatecholate
References
Frausto da Silva, J.J.R., Williams, R.J.P.: The Biological Chemistry of the Elements; The Inorganic Chemistry of Life. Clarendon Press, Oxford (1991)
Bertini, I., Gray, H.B., Lippard, S.J., Valentine, J.S.: Bioinorganic Chemistry. University Science Books, Mill Valley (1994)
Goodman Gilman, A., Goodman, L.S.: Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 10th edn. Mc-Graw Hill, New Jersey (2002)
Kimura, E., Kurogi, Y., Shionoya, M., Shira, M.: Synthesis, properties, and complexation of a new imidazole-pendant macrocyclic 12-membered triamine ligand. Inorg. Chem. 30, 4524–4529 (1991)
Schayer, R.W.: The metabolism of ring labeled histamine. J. Biol. Chem. 196, 469–475 (1952)
Baldridge, R.C., Tourtellotte, C.D.: The metabolism of histidine. III. Urinary metabolites. J. Biol. Chem. 233, 125–127 (1958)
Khandelwal, J.K., Prell, G.D., Morrishow, A.M., Green, J.P.: Presence and measurement of imidazoleacetic acid, a γ-aminobutyric acid agonist, in rat brain and human cerebrospinal fluid. J. Neurochem. 52, 1107–1113 (1989)
García-Raso, A., Fiol, J.J., Adrover, B., Tauler, P., Pons, A., Mata, I., Espinosa, E., Molins, E.: Reactivity of copper(II) peptide complexes with bioligands (benzimidazole and creatinine). Polyhedron 22, 3255–3264 (2003)
Ranford, J.D., Sadler, P.J.: Cytotoxicity and antiviral activity of transition-metal salicylato complexes and crystal structure of bis(diisopropylsalicylato)(1,10-phenanthroline)copper(II). Dalton Trans. 22, 3393–3399 (1993)
Majella, G., Vivienne, S., Malachy, M., Michael, D., Vickie, M.: Synthesis and anti-candida activity of copper(II) and manganese(II) carboxylate complexes: X-ray crystal structures of [Cu(sal)(bipy)]·C2H5OH·H2O and [Cu(norb)(phen)2]·6.5H2O (salH2 = salicylic acid; norbH2 = cis-5-norbornene-endo-2,3-dicarboxylic acid; bipy = 2,2′-bipyridine; phen = 1,10-phenanthroline). Polyhedron 18, 2931–2939 (1999)
Saha, D.K., Sandbhor, U., Shirisha, K., Padhye, S., Deobagkar, D., Ansond, C.E., Powell, A.K.: A novel mixed-ligand antimycobacterial dimeric copper complex of ciprofloxacin and phenanthroline. Bioorg. Med. Chem. Lett. 14, 3027–3032 (2004)
Zoroddu, M.A., Zanetti, S., Pogni, R., Basosi, R.: An electron spin resonance study and antimicrobial activity of copper(II)–phenanthroline complexes. J. Inorg. Biochem. 63, 291–300 (1996)
Sigel, H.: Metal Ions in Biological Systems, vol. 3. Marcel Dekker, New York (1974)
Lau, S., Sarkar, B.: Kinetic studies of copper(II)-exchange from l-histidine to human serum albumin and diglycyl-l-histidine, a peptide mimicking the copper(II)-transport site of albumin. Can. J. Chem. 53, 710–715 (1975)
Bensichem, M.V., Farrell, N.: Activation of the trans geometry in platinum antitumor complexes. Synthesis, characterization, and biological activity of complexes with the planar ligands pyridine, N-methylimidazole, thiazole, and quinoline. Crystal and molecular structure of trans-dichlorobis(thiazole)platinum(II). Inorg. Chem. 31, 634–639 (1992)
El-Sherif, A.A., Shoukry, M.M., Abd El-Gawad, M.M.A.: Protonation equilibria of some selected α-amino acids in DMSO–water mixture and their Cu(II)-complexes. J. Solution Chem. 42, 412–427 (2013)
Shoukry, M.M., Khairy, E.M., El-Sherif, A.A.: Ternary complexes involving copper(II) and amino acids, peptides and DNA constituents. The kinetics of hydrolysis of α-amino acid esters. Trans. Met. Chem. 27, 656–664 (2002)
El-Sherif, A.A., Aljahdali, M.: Equilibrium studies of binary and mixed-ligand complexes of zinc(II) involving 2-(aminomethyl)-benzimidazole and some bio-relevant ligands. J. Solution Chem. 41, 1759–1776 (2012)
El-Sherif, A.A., Shoukry, M.M., van Eldik, R.: Complex formation reactions and stability constants for mixed-ligand complexes of diaqua(2-picolylamine)palladium(II) with some bio-relevant ligands. J. Chem. Soc. Dalton Trans. 7, 1425–1432 (2003)
El-Sherif, A.A.: Mixed-ligand complexes of 2-(aminomethyl)benzimidazole palladium(II) with various biologically relevant ligands. J. Solution Chem. 35, 1287–1301 (2006)
Mahmoud, M.M.A., El-Sherif, A.A.: Complex formation equilibria between Zn(II), nitrile-tris(methyl phosphonic acid) and some bio-relevant ligands. The kinetics and mechanism for zinc(II) ion promoted hydrolysis of glycine methyl ester. J. Solution Chem. 39, 639–653 (2010)
El-Sherif, A.A.: Mixed ligand complex formation reactions and equilibrium studies of Cu(II) with bidentate heterocyclic alcohol (N, O) and some bio-relevant ligands. J. Solution Chem. 41, 813–824 (2010)
El-Sherif, A.A.: Coordination properties of bidentate (N, O) and tridentate (N, O, O) heterocyclic alcohols with dimethyltin(IV) ion. J. Coord. Chem. 64, 1240–1253 (2011)
El-Sherif, A.A., Shoukry, M.M., Hosny, W.M., Abd Al-Moghny, M.G.: Complex formation equilibria of unusual seven-coordinate Fe(EDTA) complexes with DNA constituents and related bio-relevant ligands. J. Solution Chem. 41, 813–827 (2012)
Welcher, F.J.: The Analytical Uses of Ethylenediamine Tetraacetic Acid. Van Nostand, Princeton (1965)
Gans, P., Sabatini, A., Vacca, A.: An improved computer program for the computation of formation constants from potentiometric data. Inorg. Chim. Acta 18, 237–239 (1976)
Pettit, L.D.: IUPAC Stability Constants Database. Academic Software (1993)
Perrin, D.D.: Stability Constants of Metal–Ion Complexes. Part B. Organic Ligands. Pergamon Press, Oxford (1979)
Török, I., Surdy, P., Rockenbauer, A., Korecz Jr, L., Anthony, G., Koolhaas, A., Gajda, T.: Nickel(II)-, copper(II)- and zinc(II)-complexes of some substituted imidazole ligands. J. Inorg. Biochem. 71, 7–14 (1998)
Zimmermann, S.C., Korthals, J.S., Cramer, K.D.: Syn and anti-oriented imidazole carboxylates as models for the histidine–aspartate couple in serine proteases and other enzymes. Tetrahedron 47, 2649–2660 (1991)
Figgs, B.N.: Introduction to Ligand Fields. Interscience Publishers, New York (1996)
Huheey, J.E.: Inorganic Chemistry—Principles of Structure and Reactivity. Harper SI Edn, New York (1983)
Irving, H., Williams, R.J.P.: Some factors controlling the selectivity of organic reagents. Analyst (Lond) 77, 813–829 (1952)
Cotton, F.A., Wilkinson, G.: Advanced Inorganic Chemistry. Wiley, London (1962)
Phillips, C.S.G., Williams, R.J.P.: Inorganic Chemistry, vol. 2, p. 268. Oxford University Press, Oxford (1966)
Harlly, F.R., Burgess, R.M., Alcock, R.M.: Solution Equilibria, p. 257. Ellis Harwood, Chichester (1980)
Sigel, H., Martin, R.B.: Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 82, 385–426 (1982)
Sovago, I.: In: Burger, K. (ed.) Biocoordination Chemistry. Ellis Horwood, Chichester (1990)
Pettit, L.D., Gregor, J.E., Kozlowski, H.: Perspectives in Bioinorganic Chemistry, vol. 1, pp. 1–41. Jai Press Ltd, Greenwich (1991)
El-Sherif, A.A., Shoukry, M.M.: Ternary copper(II) complexes involving 2-(aminomethyl)-benzimidazole and some bio-relevant ligands. Equilibrium studies and kinetics of hydrolysis for glycine methyl ester under complex formation. Inorg. Chim. Acta 360, 473–487 (2007)
Maskos, K.: The interaction of metal ions with nucleic acids. A nuclear magnetic resonance relaxation time study of the copper(II)–inosine-5′-monophosphate system in solution. Acta Biochim. Polonica 28(2), 183–200 (1981)
Martin, R.B., Mariam, Y.H.: Metal Ions in Biological Systems, vol. 8, pp. 57–125. Marcel Dekker, New York (1979)
Sigel, H.: Metal Ions in Biological Systems, vol. 2. Marcel Dekker, New York (1973)
Sigel, H.: Stability, structure and reactivity of mixed ligand complexes in solution. In: Banerjea, D. (ed.) Coordination Chemistry, vol. 20, pp. 27–45. IUPAC/Pergamon Press, Oxford (1980)
Sigel, H.: Ternary Cu2+ complex: stability, structure and reactivity. Angew. Chem. Int. Ed. Engl. 14, 394–402 (1975)
DeWitt, R., Watters, J.I.: Spectrophotometric investigation of a mixed complex of copper(II) ion with oxalate ion and ethylenediamine. J. Am. Chem. Soc. 76, 3810–3814 (1954)
Martin, R.B., Prados, R.: Some factors influencing mixed complex formation. J. Inorg. Nucl. Chem. 36, 1665–1670 (1974)
Munakata, M., Harada, M., Niina, S.: Stability enhancement of mixed-ligand copper(II), nickel(II), and cobalt(II) complexes with 2,2′-bipyridyl and β-diketonates. Inorg. Chem. 15, 1727–1729 (1976)
Maggiore, R., Musumeci, S., Rizzarelli, E., Sammartano, S.: Complexes of Cu2+ with 2,2′-dipyridyl and cyclohexane-1,1-dicarboxylic acid. Inorg. Chim. Acta 18, 155–158 (1976)
Walker, F.A., Sigel, H., McCormick, D.B.: Spectral properties of mixed-ligand copper(II) complexes and their corresponding binary parent complexes. Inorg. Chem. 11, 2756–2763 (1972)
Sigel, H., Huber, P.R., Griesser, R.: Ternary complexes in solution. IX. Stability-increasing effect of the pyridyl and imidazole groups on the formation of mixed-ligand copper(II)–pyrocatecholate complexes. Inorg. Chem. 10, 945–947 (1971)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aljahdali, M., El-Sherif, A.A., Shoukry, M.M. et al. Potentiometric and Thermodynamic Studies of Binary and Ternary Transition Metal(II) Complexes of Imidazole-4-acetic Acid and Some Bio-relevant Ligands. J Solution Chem 42, 1028–1050 (2013). https://doi.org/10.1007/s10953-013-0015-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0015-9