Abstract
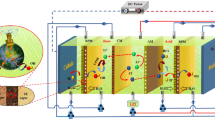
We compared the electrodialysis performance for HNO3 and NaOH recovery from NaNO3 solution by conventional electrodialysis (ED) and electrodialysis with bipolar membranes (EDBM) at constant current and constant voltage. The individual resistances of the components of the electrodialysis systems were also evaluated. The electrodialysis extent for HNO3 and NaOH recovery from NaNO3 solution was almost proportional to the total amount of electricity supplied to the system, regardless of the operation mode and the electrodialysis systems. For the same volume of feed solution, the energy consumption and current efficiency differed depending on the operation mode and the electrodialysis system. In both the ED and EDBM systems, the conductivity of the feed solution strongly affected the overall cell resistance after approximately 50% of the ions in the feed solution had migrated.
Similar content being viewed by others
References
K.-W. Kim, J.-T. Hyun, K.-Y. Lee, E.-H. Lee, D.-Y. Chung and J.-K. Moon, Ind. Eng. Chem. Res., 51, 6275 (2012).
International Atomic Energy Agency, IAEA Report, IAEA-TECDOC-1115 (1999).
K.-W. Kim, J.-T. Hyun, E.-H. Lee, G.-I. Park, K.-W. Lee, M.-J. Yoo, K.-C. Song and J.-K. Moon, J. Nucl. Mat., 418, 93 (2011).
S. M. Pepper, L. F. Brodnax, S. E. Field, R. A. Zehnder, S. N. Valdez and W. H. Runde, Ind. Eng. Chem. Res., 43, 8188 (2004).
C. F.V. Mason, W.R. J. R. Turney, B. M. Thomson, N. Lu, P. A. Longmire and C. J. Chisholm-Brause, Environ. Sci. Technol., 31, 2707 (1997).
D.-Y. Chung, H.-S. Seo, J.-W. Lee, H.-B. Yang, E.-H. Lee and K.-W. Kim, J. Radioanal. Nucl. Chem., 284, 123 (2010).
K.-W. Kim, Y.-H. Kim, S.-Y. Lee, E.-H. Lee, K.-C. Song and K. Song, Ind. Eng. Chem. Res., 48, 2085 (2009).
X. Tongwen, Resour. Conserv. Recycl., 37, 1 (2002).
Y. Wang, N. Zhang, C. Huang and T. Xu, J. Membr. Sci., 385–386, 226 (2011).
V. Cauwenberg, J. Peels, S. Resbeut and G. Pourcelly, Sep. Purif. Technol., 22–23, 115 (2001).
M. Fidaleo and M. Moresi, Biotech. & Bioeng., 91(5), 556 (2005).
B. Bauer, F. J. Gerner and H. Strathmann, Desalination, 68, 279 (1988).
K. N. Mani, J. Membr. Sci., 58, 117 (1991).
F. Schaffner, P.-Y. Pontalier, V. Sanchez and F. Lutic, Desalination, 170, 113 (2004).
A. Tanioka, K. Shimizu, T. Hosono, R. Eto and T. Osaki, Colloids Surf. A: Physicochem. Eng. Aspects, 159, 305 (1999).
K. Venugopal and S. Dharmalingam, Desalination, 296, 37 (2012).
M. Sadrazdeh and T. Mohammadi, Desalination, 249, 279 (2009).
T. Sata, K. Mine and M. Higa, J. Membr. Sci., 141, 137 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KW., Hyun, JT., Lee, KY. et al. Evaluation of recovery characteristic of acidic and alkaline solutions from NaNO3 using conventional electrodialysis and electrodialysis with bipolar membranes. Korean J. Chem. Eng. 30, 1760–1769 (2013). https://doi.org/10.1007/s11814-013-0089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0089-5