Abstract
A feasibility study was carried out on generation of hydrochloric acid and lithium hydroxide from the simulated lithium chloride solution using EX3B model bipolar membrane electrodialysis (BMED). The influence of a series of process parameters, such as feed concentration, initial acid and base concentration in device component, feed solution volume, and current density were investigated. In addition, the maximum achievable concentrations of HCl and LiOH, the average current efficiency, and specific energy consumption were also studied and compared in this paper to the existing literature. Higher LiCl concentrations in the feed solution were found to be beneficial in increasing the final concentrations of HCl and LiOH, as well as improving current efficiency while decreasing specific energy consumption. However, when its concentration was less than 4 g/L, the membrane stack voltage curve of BMED increased rapidly, attributed to the higher solution resistance. Also low initial concentration of acid and base employed in device component can improve the current efficiency. Increasing of the initial concentration of acid and base solution lowered energy consumption. Moreover, a high current density could rapidly increase HCl and LiOH concentration and enhance water movements of BMED process, but reduced the current efficiency. The maximum achievable concentration of HCl and LiOH generated from 130 g/L LiCl solution were close to 3.24 mol/L and 3.57 mol/L, respectively. In summary, the present study confirmed the feasible application for the generation of HCl and LiOH from simulated lithium chloride solution with BMED.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Lithium is the lightest metal and the least dense solid element in nature. In recently years, lithium and its compounds have a much-diversified industrial applications owe to their special physical and chemical properties. Lithium hydroxide, in particular, is one of the most important lithium feeds, which constitutes a raw material in many commercial applications, including well-known lithium-ion batteries [3, 17], lubricating greases [2], chemical reagents, ceramics and glasses [4]. Owing to the rapid development of the economy and industrialization, it is estimated that the global demand for lithium hydroxide will be 546,400 tons in 2025, and the CAGR will be about 55.28%. Lithium hydroxide is mostly produced from brines or lithium-containing ores. It is difficult to produce battery grade lithium hydroxide via brine resources straightaway. It often requires to convert lithium carbonate to lithium hydroxide using a causticization method [24]. Unfortunately, the cost of causticization method is greatly influenced by the price of lithium carbonate and the upfront capital cost of the required is relatively high. The limestone roasting method, as a traditional preparation process, also has some disadvantages of high energy consumption and heavy environmental pollution. On other side, the roasting method [1, 12] employs a large number of chemicals, which potentially can introduce other impurities into final products. More recently, battery manufacturers such as Tesla, BYD, BAIC and Daimler have put forward higher requirements on the quality of battery grade lithium hydroxide [33].
Driven by the market demanding, many researchers have made continuous efforts toward improving the quality of lithium hydroxide and reducing energy consumption. Electrodialysis (ED) is emerging as one of the most promising technologies has been investigated as an alternative since 2003s [10, 24]. However, no industrial application has been found so far due to its high capital costs and technical obstacles. In the last a few years, bipolar membrane electrodialysis technique was developed and has attracted intensive research attention due to its environmental benignity and energy efficiency [32].
BMED is an electrodialysis method in combination with a bipolar membrane, which can enhance ionic mobilities under the direct current and limit the transmembrane migration of ions by ion selective exchange membrane. It is widely applied in the food, chemical industry and effluent treatment. Fruitful research has been performed using BMED to separate ions from neutral solution and convert them into acid and base [8, 25]. Moreover, the production of sodium hydroxide through BMED from reverse osmosis concentrate [9, 35], glyphosate neutralization liquor [27, 34], brominated butyl rubber wastewater [32] and other industrial wastewater [14, 31] have been reported, the results showed that it could be used as a promising and environmentally friendly technique to regeneration sodium hydroxide. The desalination rate of BMED for industrial NH4Cl wastewater was 92%, and HCl and NH3·H2O concentrations obtained from the simulated NH4Cl solution was up to 1.58 M and 1.3 M, respectively[16]. These reports presented that BMED technique played an extremely important role in generation of inorganic acids and bases and the treatment of industrial high-salinity brine. However, reports about the production of HCl and LiOH from Lithium ore leaching solution such as lithium chloride solutions have not been found so far. Therefore, the focus of the research work in this paper was to explore whether BMED technology could be applied in industry as an alternative to traditional technique for the production of lithium hydroxide from lithium ore leaching solution, and to observe the energy consumption and the factors affecting it.
In the present work, simulated LiCl solution was treated by a EX3B model BMED device for the generation of hydrochloric acid and lithium hydroxide. The effects of process conditions were investigated. Moreover, the water movement in each compartment and the mechanism of affecting the maximum concentration of HCl and LiOH were discussed. The present study focused on analyzing the process phenomena and differences of HCl and LiOH generated from simulated LiCl solution, aiming for verifying the potential application of BMED for the regeneration of LiOH for the production lithium ion battery.
2 Experimental methods
2.1 Reagents and membranes
Hydrochloric acid (Greagent, 36%-38%), Lithium hydroxide monohydrate (LiOH, solid, ≥ 99.5%), lithium chloride (solid, ≥ 99.5%), sodium sulfate (Adamas-beta®, ≥ 99.5%). Sodium hydroxide (Adamas-beta®, ≥ 98%), silver nitrate (Adamas-beta®, ≥ 99%), phenolphthalein (IND, Greagent) and potassium chromate (Sigma-Aldrich, ≥ 99.5%) were used in the experiment. the reagents of Lithium hydroxide monohydrate and lithium chloride were produced by Tianqi lithium Co., Ltd, Other reagents were purchased from Shanghai Titan Scientific Co., Ltd.
The ions membranes used in the present experiments were BPM-1 (bipolar membranes, ASTOM Co. Ltd, Japan), CM-4 (cation membranes, ASTOM Co. Ltd, Japan) and AM-4 (anion membranes, ASTOM Co. Ltd, Japan). All of the membranes were purchased from Hangzhou Lanran Environmental Technology Co., Ltd (Hangzhou, China). The properties of three kinds of membranes were presented in Tables 1 and 2.
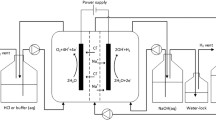
A lab-scale bipolar membrane electrodialysis apparatus (EX3B) was provided by Hangzhou Lanran Environmental Technology Co., Ltd, China. And it was installed according to the BPM-AM-CM-BPM configuration (including acid, base and feed compartments) in the experiment shown in Fig. 1. The membrane stack is comprised of (a) electrodes, which are made of titanium electrode coated with ruthenium and iridium, (b) membranes, each membrane had an effective area of 55 cm2 and there are 10 membrane triples in a membrane stack. The spacer channel width was 0.75 mm. The BMED system was connected to an DC power. Driven by three peristaltic pumps, the acid solution (0.05 mol/L HCl, 700 mL), base solution (0.05 mol/L LiOH, 700 mL) and feed solution (70-130 g LiCl/L, 700 mL) and electrode rinse solution (0.5 mol/L Na2SO4, 700 mL) were circulated in three-compartments system, respectively. Unless otherwise noted, the initial volume of each compartment was 700 mL and the current density was 60 mA/cm2.
In this experiment, lithium chloride solution was pumped to a membrane stack as the feed, Li+ and Cl− ions in feed compartment migrated through cation membrane into base compartment and anion membranes into acid compartment under a DC voltage, respectively, and then lithium ion and chloride ions combined with hydroxide ions and hydrogen ions generated from water splitting to produce LiOH and HCl, respectively.
2.2 Chemical analysis and calculations
2.2.1 Chemical analysis
The Li+ ions concentration was analyzed by Atomic Absorption Spectrometry (AA-7050, Shanghai Yoke Instrument Co., Ltd, China). The Cl− ions concentration was determined by using standard silver nitrate solution as titrant and potassium chromate as indicator. The HCl concentration was determined by titration with standard sodium hydroxide solution using phenolphthalein as indicator.
2.2.2 Data processing
The average current efficiency (ƞ) and unit energy consumption (EC) are important standards for evaluation of the electrodialysis performance [36]. The (ƞ) is the ratio of practically obtained molar amount of acid/base to that which should theoretically be obtained. The calculation equation is as follows (1) [29]:
where z is the ion valence. F is Faraday constant (96,485 C/mol). C0 and Ct are the concentration (mol/L) of HCl or LiOH at time 0 and t (min), respectively. V0 and Vt are the volume (L) at time 0 and time t (min). N, \(\Delta t\) and I are the number of cell triplets (N = 10), time period (min) and current (A), respectively.
The calculation equation of unit energy consumption ( kWh/kg) as follows (2) [29]:
where t is the time (min). U is the stack voltage (V) at time t (min). M stands for the molar mass (g/mol) of HCl or LiOH, pumping energy consumption has not been considered.
The liner velocities of acid, base, and feed solution in the compartments are 3.3 cm/s and its calculation equation as follows (3)[16]:
where V (cm/s) is the liner velocities, W(cm) and d(cm) stand for width of flow section area (5 cm) and spacer channel width (0.75 mm), respectively. Q (L/h) is the flow rate of acid, base or feed solution (45 L/h).
3 Results and discussion
3.1 Effect of initial LiCl concentration in feed compartment
In order to examine the influence of the feed concentration, three different concentrations including 70 g/L, 100 g/L and 130 g/L were employed.
Figure 2 presents the variation of stack voltage and Cl− concentration at the constant-current density of 60 mA/cm2. It can be observed clearly that the voltage curve of the membrane stack presented three trends as time goes on: falling stage, steady stage, and rising stage. This phenomenon was considered to be closely related to the total resistance of membrane stack at a constant current in BMED system (including the resistance of membranes and solution). At the beginning stage, the voltage curve dropped gradually indicated that the solution resistance decreased in compartment due to the H2O molecules at the interphase layer of the BPM were split into H+ and OH− ions, and ions migration from feed solution into acid and base compartments through the anion membrane and cation membrane, respectively. As the experiment progresses, the membrane stack voltage roughly constant for a period of time because the total stack resistance remained unchanged. However, the membrane stack voltage curve increased sharply at the later stage of the experiments, this phenomenon can be explained by the depletion of LiCl in feed compartment (the content of ions dropped to an extremely low level), which lead to the high resistance of the membrane stack. So the changes of membrane stack voltage were closely associated with the increases of acid and base concentration in the beginning of the experiment and the ions depletion in feed solution at the later stage of experiments.
When the membrane voltage of the BMED system rises rapidly, the samples of feed solution in feed compartment were taken out and the Cl− ions concentration was analyzed. It can be observed from Fig. 2 that the LiCl concentration contained in feed compartment dropped to less than 4 g/L, which was consistent with the "inflection point" of membrane stack voltage. That indicated that when the LiCl concentration contained in feed compartment was less than 4 g/L, the BMED system is no longer suitable for treatment because the lower ionic content could lead to the higher solution resistance, which resulted in the higher energy consumption of BMED system. The low-concentration LiCl solution can be further concentrated by other membrane equipment to achieve reuse purposes. while the concentrated solution obtained could also be used to produce HCl and LiOH with BMED.
The concentration of HCl and LiOH solution with different feed concentrations were compared, and the results were shown in Fig. 3. It was found that the HCl and LiOH concentration increased generally as time elapsed. As it can be seen, when the feed solution containing LiCl of 70 g/L, 100 g/L, and 130 g/L after continuously running for 80, 120 and, 180 min, the HCl and LiOH concentration were 1.41 mol/L, 1.83 mol/L, 2.24 mol/L and 1.26 mol/L, 1.71 mol/L, 2.06 mol/L, respectively. The HCl solution obtained in acid compartment could meet the requirements of the ion exchange resin regeneration [38, 39] and the cleaning of reverse osmosis membrane [18, 20, 21]. Moreover, the HCl solution can also be used for more applications by evaporation or other methods, and the LiOH solution obtained can be used to produce battery-grade lithium hydroxide monohydrate (LiOH·H2O) products by evaporation and recrystallization. Figure 3 shows that the concentration of HCl and LiOH at the initial stage increased faster than that at the later stage, which was caused by the decrease of ionic content and water electricity penetration [28]. The decrease of ionic content in feed compartment would inevitably result in less Li+ and Cl− ions migrating from feed compartment to the adjacent compartments. Furthermore, Cl− and Li+ ions in the form of hydration ions migrated from feed compartment into the adjacent compartment through the anion membrane and cation membrane, respectively, which resulted in the increase of acid and base solution volume (the volume variation was shown in Fig. 4). Therefore, the growth rate of HCl and LiOH concentration were delayed. These results meant that increasing the concentration of LiCl in feed compartment was helpful to obtain higher acid and base concentrations.
Figure 4 shows the variation of solution volume in feed, acid, and base compartments with different feed concentrations. The solution volume in the feed compartment decreased linearly while the acid and base volume increased linearly as time elapsed. The reduced volume of solution in feed compartment was roughly equal to the sum of the volumetric increment of solution in acid and base compartment, which was consistent with the research results of Lizuka [7]. It can be observed from Fig. 4 that there was a significant difference in the slope of linearized curves, indicating that the change rate of solution volume in feed, acid and base compartments decreased with the increase of the initial feed concentration. The volume variation of solution in acid, base and feed compartments was mainly due to water movements driven by the concentration diffusion and electro-osmosis (counter-ions migration) [11, 19]. The electro-osmosis influence on water migration was dominant, which could drive the counter-ions in the form of hydrated ions to migrate into the acid and base compartments. Therefore, the volume of feed compartment displayed a decreasing trend while that of acid and base compartments increasing. Furthermore, it was also observed that the volume of base compartment increased faster than the volume of the acid compartment. This phenomenon was closely related to the number of ions transferred across the membranes, which is higher in the cation membrane.
Figure 5 shows that the variation of pH and conductivity of feed solution with different initial LiCl concentration. It can be observed that the conductivity of feed solution decreased gradually to 0 at the end of the experiments, which indicated that the ions in the feed solution were almost depleted as time elapsed. However, lower concentration of LiCl solution was not conducive to the concentration increase of HCl and LiOH. It was also proved that the simulated LiCl solution can realize the goal of the generation of hydrochloric acid and lithium hydroxide monohydrate and desalination simultaneously by BMED. Figure 5a indicated that the initial LiCl solution was always weakly alkaline. While the pH dropped sharply in feed solution might be due to the higher H+ ions leakage though the anion membrane at the beginning of the experiment, According to the literature [5, 23, 37] protons can be easily transported across anion membrane from acid compartment into adjacent feed compartment by the so-called tunnel mechanism. When the process ended, the increase in the pH value of feed solution might be ascribed to the concentration polarization phenomenon, because the LiCl concentration contained in the feed compartment was too low at that period [13].
Figure 6 demonstrated the influence of the feed concentration on the current efficiency (ƞ) and unit energy consumption (EC) when the current density was 60 mA/cm2. It was found that the higher initial concentration of LiCl was beneficial to increase ƞ and reduce EC. According to the literature, the EC was 5.5 kWh/kg for the acid (H2SO4) and 4.8 kWh/kg for the base (NaOH) with the initial concentration of 75 g Na2SO4/L at the constant current density of 60 mA/cm2[29], and the EC of 60 g/L NH4Cl was treated by BMED was 9.1 kWh/kg for NH3·H2O and 8.5 kWh/kg for HCl when current density was 48 mA/cm2 [16], while EC of the acid (HCl) and base (LiOH) with the initial concentration of 70 g LiCl/L demonstrated in Fig. 6 were 2.2 and 3.7 kWh/kg, respectively, which are much lower than those reported in the literature [16, 29]. At a current density of 30 mA/cm2, the ƞ of 100 g/L NaCl was about 65% for the base (NaOH) and 84% for the acid (HCl) with initial acid and base concentration of 0.05 M [22], which were lower than the ƞ values in Fig. 6. One possible explanation for these results stems from the fact that the dissolution of lithium chloride in water was an exothermic reaction and the present room temperatures are above 28 °C during the summer season. The higher temperature of the initial LiCl solution in salt compartment led to the lower EC and higher ƞ in the BMED process. Although the influence of temperature on electrodialysis performance is complex because the mass transfer process involved, these phenomena indicated that the mobility of ions in adjacent compartment increases with the increase of temperature probably, which had a significant impact on the decrease in the resistance of the membrane stack. Additionally, the EC and ƞ were also associated with other factors, such as operating conditions and characteristics of membranes (BM, AEM, and CEM) [25, 30].
3.2 Influences of initial concentration of HCl and LiOH
In order to have a lower resistance in membrane stack, HCl and LiOH were added to the acid and base compartments to investigate the effect of initial concentration on generation of HCl and LiOH when the current density was 60 mA/cm2, and results are shown in Fig. 7. The HCl and LiOH concentration increased slightly with the increase of initial concentration of acid and base, while the growth of HCl and LiOH concentration was inhibited by the higher initial concentration of acid and base compartments. Which might be resulted from the leakage of H+ and OH− ions through the ions membrane. At the beginning of the experiment, more H+ and OH− ions in the initial acid and base compartments resulted in more ions leakage. After that, the concentration gradient of feed and acid/base compartments increased gradually as the feed concentration decreased and the acid/base concentration increased, which resulted in the high driving force for the ion’s reverse diffusion according to Fick's first law. Furthermore, this can also be explained that the higher initial concentration of HCl and LiOH could lead to the increase of transfer resistance from feed compartment into acid or base compartment, which could inhibit the increase of HCl and LiOH. Therefore, increasing initial concentration of acid and base maybe not a good way to improve the yield of HCl and LiOH generated from LiCl solution by BMED.
Figure 8 illustrates the current efficiency (ƞ) and energy consumption (EC) curves of BMED at different initial concentrations. It was found that the EC of HCl and LiOH has no significant increase as the increase of initial acid and base concentration, but a slight decrease. The reason for this was that a higher initial concentration of acid and base will inevitably lead to a decrease in the membrane stack resistance since the increase of initial conductivity in acid and base solution. However, with the initial concentration of acid and base compartment increasing further, the ratio of membrane stack resistance to the total resistance decreased greatly. Therefore, the EC of acid and base decreased in a much smaller magnitude in BMED system. In terms of the ƞ curve, which had been reduced from 70 to 60% for the acid (HCl) and 61% to 47% for the base (LiOH) by increasing the initial concentration of acid and base solution from 0.05 mol/L to 0.5 mol/L after running 120 min. This was caused by the fact that the growth rate of HCl and LiOH concentration was inhibited by the higher initial concentration of acid and base in device component.
3.3 Effects of current density
The current density of acid and base regeneration is an important influence factor in the BMED. The influence of different current densities on the concentrations of HCl and LiOH were examined when the initial concentration was 100 g LiCl/L. It was found from Fig. 9 that the concentration of HCl and LiOH increased with the current density, and this growth pattern coincides with the NH4Cl system in literature [16], This is logical because the velocity of water dissociation by BPM has a dominant role in the concentration of HCl and LiOH. The velocity of water molecules were split into H+ and OH− were accelerated with the increase of current density due to the Second Wien effect [6], a larger amount of Cl− and Li+ in feed compartment were transferred to the adjacent compartment combined with protons and hydroxide ions to generate HCl and LiOH, respectively. Therefore, a higher concentration of HCl and LiOH was obtained at a higher current density. In addition, it can also be observed that the increase in current density was favored to shorten the operating time. This means that appropriately increasing the current density could effectively improve the performance of the BMED process, which can shorten the operation time and reduce ion’s reverse diffusion at the end of the experiments. As for LiOH concentration, there was a slight decrease at the end of experiment when the current density was 80 mA/cm2 (compared to 60 mA/cm2), Furthermore, the concentration of LiOH exhibited a slower increment at the later stage of the experiment, which resulted from the leakage of OH– from base compartment into acid compartment. However, the molar concentration of HCl produced was always slightly lower than the corresponding concentration of LiOH. This was attributed to the fact that H+ has greater inherent mobility than OH−, which resulted in more H+ with a smaller radius of hydrated ions, leaking from the acid compartment to the feed compartment [15, 26]. In addition, current density had a greater impact on water migration. As shown in Fig. 9 (right), the degree of water migration increased as the current density increased. Therefore, when a larger current density was applied, desalinated solution of a smaller volume and a larger volume of acid and base solution can be obtained within the same experimental time.
Figure 10 demonstrates the influence of current density on the ƞ and EC of BMED process. It can be observed that the lower current density was helpful to increase the ƞ and reduce EC of HCl and LiOH generated from LiCl, which was completely opposite to the trend of different feed concentrations shown in Fig. 6. Furthermore, there was a tiny difference between the ƞ curves of 60 mA/cm2 and 80 mA/cm2. However, the gap increased significantly when the current density dropped to 40 mA/m2. For example, when the operating time was 80 min and current density were 40, 60 and 80 mA/cm2, η, and EC for LiOH were 0.8 and 2.38 kWh/kg, 0.65 and 3.31 kWh/kg, and 0.62 and 3.78 kWh/kg, respectively. To sum up, this phenomenon was mainly related to the transmembrane migration of ions in adjacent compartments and the dissociation of H2O molecules at the interphase layer of the BPM. The higher the current density was, the faster the velocity of ions flux was. When the ions adsorbed on the membrane were depleted, it would directly cause the increase of stack resistance and even H2O molecules splitting. Moreover, the higher current density was also helpful to inhibit ions unexpected transfer, especially at the later stage of the experiments. The energy consumption curve of HCl and LiOH increased gradually with the increase of current density during BMED process, which resulted from that a large part of the electricity was consumed to overcome the membrane stack resistance. Therefore, the appropriate high current density should be selected for the BMED system when considering daily acid and base output and the process cost.
3.4 Effects of feed volume and the maximum achievable concentration of HCl and LiOH
The lower concentration of LiCl in feed compartment would greatly affect the final concentration of HCl and LiOH, especially at the end of the experiments when LiCl concentration dropped to a much lower level. Therefore, in order to further investigate the effect of initial feed solution volume on a generation of HCl and LiOH, the experiments of 70 g/L LiCl were performed with the different initial feed solution volumes of 700 mL and 2100 mL in BMED system.
Figure 11 illustrates that the influence of different initial volumes of LiCl solution on concentration of HCl and LiOH. It could be seen from Fig. 11 that the concentration of HCl and LiOH were 2.52 mol/L and 2.5 mol/L at the initial feed volume of 2100 mL, which were much higher than that with the initial volume of LiCl solution was 700 mL. This was caused by the fact that the higher total mole number of LiCl in feed compartment necessarily led to the increase of Li+ and Cl− ions from feed compartment to transmembrane migrate into the adjacent compartment in BMED process. Furthermore, it was found that the concentration curve of V feed: V acid or base = 1:1 did not completely coincide with the curve of Vfeed: Vacid or base = 3:1 in the period range 40 ~ 80 min. Which mainly due to less ion migration and intensification of the concentration difference polarization phenomenon when the initial feed volume was 700 mL because the LiCl concentration in feed compartment was too low at that period. Therefore, it may be an effective method to increase the final concentration of HCl and LiOH generated from LiCl solution by increasing the initial volume of feed compartment or increasing initial concentration of feed compartment.
Figure 12 demonstrates the influence of initial feed volume on the efficiency and unit energy consumption. Increasing the initial volume of feed solution was helpful to improve the performance of electrodialysis process, because it can increase efficiency and reduce energy consumption of HCl and LiOH generated from LiCl solution. It was found that when the initial feed volume increased from 700 to 2100 mL, the current efficiency of the BMED process had been improved from 61 to 66% for acid (HCl) and 61% to 80% for base (LiOH), respectively, while the unit energy consumption of LiOH had been reduced from 2.1 to 1.6 kWh/kg after run 80 min. The main reason should be that the content of ions in feed compartment had no significant change after run 80 min when the feed solution volume was 2100 mL, in contrast with the feed solution volume of 700 mL, which had a significant decrease in ions content of feed solution when the total mole number of LiCl in the salt solution dropped to a much lower level. Therefore, an appropriate increase in the solution volume of feed was helpful to generate a higher concentration of HCl and LiOH with BMED.
The maximum achievable concentration of HCl and LiOH was also considered to be the important parameter to evaluate the performance of membranes in BMED system. In addition to the limitations of the bipolar membrane, for a given application, the selection of anion exchange membranes was also the most important since their inferior permselectivity and chemical stability compared with cation ion exchange membranes.
In order to maintain a constant concentration of LiCl in feed compartment during the experiment, a higher concentration of lithium chloride solution was added continuously to feed compartment to balance the decrease of concentration and volume in the feed compartment by a peristaltic pump. Figure 13 shows that the maximum achievable concentration of HCl and LiOH with the initial concentration of 130 g LiCl/L at the current density of 80 mA/cm2. It can be observed from Fig. 13 that the growth rate of HCl and LiOH concentration at the end of the experiment was significantly lower than that in the initial stage, resulted from the ions reverse diffusion and the increase of ions transfer resistance. The maximum concentration of LiOH generated from LiCl was about 3.58 mol/L after continuously running for 360 min. However, the maximum concentration of HCl was slightly lower than the corresponding concentration of LiOH. It was also proved that the maximum concentration of HCl and LiOH obtained from LiCl solution were mainly determined by the properties of the anion ions exchange membrane which has limited retention of protons since the so-called proton-tunneling mechanism. Similarly, a higher concentration of hydroxide ions can permeate from the base compartment through the cation ions membrane, but to a lesser extent. The net result is that the hydrogen ions and hydroxide ions generated from the splitting of water migrating from the acid or base compartment through the ions membrane to neutralize each other in feed compartment. Which resulted in the lower current utilization of BMED process. Therefore, the selection of an appropriate output concentration of HCl and LiOH was favor to reduce the leakage and reverse diffusion of ions.
4 Conclusions
A lab-scale BMED system for the generation of HCl and LiOH from simulated lithium chloride solution was presented in the study. The influence of regents and operating parameters were investigated. The results indicate that the higher initial concentration of LiCl was beneficial to increase the concentration of HCl and LiOH and average current efficiency, while decreasing the specific energy consumption of HCl and LiOH. The stack voltage increased rapidly when the LiCl concentration within the feed compartment was less than the “inflection point concentration” about 4 g/L. when changing the initial volume of 70 g/L LiCl from 700 to 2100 mL, the concentration of HCl and LiOH increase from 1.31 mol/L and 1.26 mol/L to 2.53 mol/L and 2.52 mol/L, respectively, while the unit energy consumption had been reduced from 3.68 kWh/kg to 2.81 kWh/kg for the base (LiOH) after run 80 min. Moreover, a high current density could rapidly increase HCl and LiOH concentration and enhance water movements of BMED process, but reduce the current efficiency.
The higher initial concentration of acid and base was in favored to improve the final concentration of them, but an excessively high initial concentration was adverse to the growth of HCl and LiOH concentration. The maximum concentration of HCl and LiOH were close to 3.24 mol/L and 3.57 mol/L, respectively, obtained at a constant concentration of 130 g LiCl/L and the current density of 80 mA/cm2. The produced HCl can potentially be used for resin regeneration and lithium chloride conversion from lithium sulfate, and LiOH can be treated for the production of battery-grade LiOH. In summary, the results confirm the feasibility for the generation of HCl and LiOH from simulated LiCl solution by BMED and provide a favorable reference for the optimization of process parameters.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Barbosa LI, González JA, Ruiz MDC (2015) Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim Acta 605:63–67. https://doi.org/10.1016/j.tca.2015.02.009
Chaduneli ZK, Shibryaev SB, Esitashvili VA, Fuks IG (1987) Influence of alkalinity on effectivness of additives in lithium greases. Chem Tech Fuels Oil 23:28–30. https://doi.org/10.1007/BF00725642
Dong T, Zhang L, Yu X, Zhao Z, Dong Y (2011) Synthesis and characterization of gradient cathode material for lithium-ion batteries. Adv Mater Res 391–392:1435–1439. https://doi.org/10.4028/www.scientific.net/AMR.391-392.1435
Esaka T, Adachi Y (2014) Electrode property of sintered ceramic based on CaMnO3 in LiOH aqueous solution. J Mater Sci Chem Eng 02:15–21. https://doi.org/10.4236/msce.2014.24002
Fu L, Gao X, Yang Y, Fan A, Hao H, Gao C (2014) Preparation of succinic acid using bipolar membrane electrodialysis. Sep Purif Technol 127:212–218. https://doi.org/10.1016/j.seppur.2014.02.028
Huang C, Xu T, Feng H, Li Q (2009) Win?Win coupling in electrodialysis with bipolar membranes (EDBM) for cleaner production. Ind Eng Chem Res 48:1699–1705. https://doi.org/10.1021/ie801192k
Iizuka A, Yamashita Y, Nagasawa H, Yamasaki A, Yanagisawa Y (2013) Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep Purif Technol 113:33–41. https://doi.org/10.1016/j.seppur.2013.04.014
İpekçi D, Altıok E, Bunani S, Yoshizuka K, Nishihama S, Arda M, Kabay N (2018) Effect of acid-base solutions used in acid-base compartments for simultaneous recovery of lithium and boron from aqueous solution using bipolar membrane electrodialysis (BMED). Desalination 448(2018):69–75. https://doi.org/10.1016/j.desal.2018.10.001
Ibáñez R, Pérez-González A, Gómez P, Urtiaga AM, Ortiz I (2013) Acid and base recovery from softened reverse osmosis (RO) brines. Exp Assess Using Model Concentr Desalinat 309:165–170. https://doi.org/10.1016/j.desal.2012.10.006
Jiang C, Wang Y, Wang Q, Feng H, Xu T (2014) Production of lithium hydroxide from lake brines through electro-electrodialysis with bipolar membranes (EEDBM). Ind Eng Chem Res 53:6103–6112. https://doi.org/10.1021/ie404334s
Jiang C, Wang Q, Li Y, Wang Y, Xu T (2015) Water electro-transport with hydrated cations in electrodialysis. Desalination 365:204–212. https://doi.org/10.1016/j.desal.2015.03.007
Kuang G, Li H, Hu S, Jin R, Liu S, Guo H (2015) Recovery of aluminium and lithium from gypsum residue obtained in the process of lithium extraction from lepidolite. Hydrometallurgy 157:214–218. https://doi.org/10.1016/j.hydromet.2015.08.020
Krol JJ, Wessling M, Strathmann H (1999) Concentration polarization with monopolar ion exchange membranes: current-voltage curves and water dissociation. J Membrane Sci 162:145–154. https://doi.org/10.1016/S0376-7388(99)00133-7
Kroupa J, Kincl J, Cakl J (2015) Recovery of H2SO4 and NaOH from Na2SO4 by electrodialysis with heterogeneous bipolar membrane. Desalin Water Treat 56:3238–3246. https://doi.org/10.1080/19443994.2014.980972
Lv Y, Yan H, Yang B, Wu C (2018) Bipolar membrane electrodialysis for the recycling of ammonium chloride wastewater: Membrane selection and process optimization. Chem Eng Res Des 138:105–115. https://doi.org/10.1016/j.cherd.2018.08.014
Li Y, Shi S, Cao H, Wu X, Zhao Z, Wang L (2016) Bipolar membrane electrodialysis for generation of hydrochloric acid and ammonia from simulated ammonium chloride wastewater. Water Res 89:201–209. https://doi.org/10.1016/j.watres.2015.11.038
Liu T, Liu Z, Kim G, Frith J, Grey C (2017) Understanding LiOH chemistry in a ruthenium catalyzed Lim2 battery. Angew Chem Int Ed 56(50):16057–16062. https://doi.org/10.1002/anie.201709886
Madaeni SS, Mohamamdi T, Moghadam MK (2001) Chemical cleaning of reverse osmosis membranes. Desalination 134:77–82. https://doi.org/10.1016/S0011-9164(01)00117-5
Mier MP, IbanEz R, Ortiz I (2008) Influence of ion concentration on the kinetics of electrodialysis with bipolar membranes. Sep Purif Technol 59: 197–205. https://doi.org/10.1016/j.seppur.2007.06.015
Madaeni SS, Samieirad S (2010) Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 257:80–86. https://doi.org/10.1002/apj.387
Madaeni SS, Mansourpanah Y (2004) Chemical cleaning of reverse osmosis membranes fouled by whey. Desalination 161:13–24. https://doi.org/10.1016/S0011-9164(04)90036-7
Reig M, Casas S, Valderrama C, Gibert O, Cortina JL (2016) Integration of monopolar and bipolar electrodialysis for valorization of seawater reverse osmosis desalination brines: Production of strong acid and base. Desalination 398:87–97. https://doi.org/10.1016/j.desal.2016.07.024
Rottiers T, Ghyselbrecht K, Meesschaert B, Van DB, Pinoy B (2014) Influence of the type of anion membrane on solvent flux and back diffusion in electrodialysis of concentrated NaCl solutions. Chem Eng Sci 113:95–100. https://doi.org/10.1016/j.ces.2014.04.008
Ryabtsev AD, Nemkov NM, Kotsupalo NP, Serikova LA (2004) Preparation of high-purity lithium hydroxide monohydrate from technical-grade lithium carbonate by membrane electrolysis. Russ J Appl Chem 77(7):1108–1116. https://doi.org/10.1023/B:RJAC.0000044158.61704.93
Bunani S, Arda M, Kabay N, Yoshizuka K, Nishihama S (2017) Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 416:10–15. https://doi.org/10.1016/j.desal.2017.04.017
Shen J, Huang J, Liu J, Ye W, Lin J, Bart VDB (2013) The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge. J Hazard Mater 260(2013):660–667. https://doi.org/10.1016/j.jhazmat.2013.06.028
Shen J, Huang J, Ruan H, Wang J, Bart VDB (2014) Techno-economic analysis of resource recovery of glyphosate liquor by membrane technology. Desalination 342:118–125. https://doi.org/10.1016/j.desal.2013.11.041
Tanaka Y (2004) Overall mass transport and solution leakage in an ion-exchange membrane electrodialyzer. J Membrane Sci 235(1–2):15–24. https://doi.org/10.1016/j.memsci.2004.01.013
Tran ATK, Mondal P, Meesschaert LJY, B, Pinoy L, Bart VDB, (2015) Simultaneous regeneration of inorganic acid and base from a metal washing step wastewater by bipolar membrane electrodialysis after pretreatment by crystallization in a fluidized pellet reactor. J Membrane Sci 473:118–127. https://doi.org/10.1016/j.memsci.2014.09.006
Wang X, Wang Y, Zhang X, Feng H, Xu T (2013) In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour Technol 147:442–448. https://doi.org/10.1016/j.biortech.2013.08.045
Wei Y, Li C, Wang Y, Xu Z, Li Q, Xu T (2012) Regenerating sodium hydroxide from the spent caustic by bipolar membrane electrodialysis (BMED). Sep Purif Technol 86:49–54. https://doi.org/10.1016/j.seppur.2011.10.019
Wei Y, Wang Y, Zhang X, Xu TW (2013) Comparative study on the treatment of simulated brominated butyl rubber wastewater by using bipolar membrane electrodialysis (BMED) and conventional electrodialysis (ED). Sep Purif Technol 110:164–169. https://doi.org/10.1016/j.seppur.2013.03.028
Weimer L, Braun T, Hemdt AV (2019) Design of a systematic value chain for lithium-ion batteries from the raw material perspective. Resour Policy 64:101473–101473. https://doi.org/10.1016/j.resourpol.2019.101473
Wang X (2013) The feasible study on thereclamation of the glyphosate neutralization liquor by bipolar membrane electrodialysis. Dissertation, Ocean University of China
Yang L, Yao J, Wang J (2017) Desalination of concentrated wastewater from reverse osmosis by bipolar membrane electrodialysis. Water Treat 98:108–114. https://doi.org/10.5004/dwt.2017.21704
Yang Y, Gao X, Fan A, Fu L, Gao C (2014) An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J Membr Sci 449:119–126. https://doi.org/10.1016/j.memsci.2013.07.066
Zhao Y, Wang H, Li Y, Wang M, Xiang X, An integrated membrane process for preparation of lithium hydroxide from high Mg/Li ratio salt lake brine. Desalination 493 :114620–114633, https://doi.org/10.1016/j.desal.2020
Zhan Y, Yu J (2010) Cause analysis and solving countermeasure of the acid and alkali consumption in the regeneration of ion exchange resin in the primary defeeding system. Industr Water Treatment 30:81–83. https://doi.org/10.1080/14697688.2012.738930
Zhang W (2013) Influence of regeneration method on regeneration degeree of cation exchange resins. Thermal Power Gener 42(2013):88–89. https://doi.org/10.3969/j.issn.1002-3364.2013.03.088
Acknowledgements
The authors would like to thank Mr. Chuan Xu, for to provide the necessary support in this work. Also, we thank to PhD Fu Zhou and PhD Chao Li for their help in data analysis and revision of work.
Funding
This work was supported by the National Key R&D Program of China (G20200136011) and Sichuan Science and Technology Program (2019YFQ0027, 2020YFS0547 and 2020ZHCG0024).
Author information
Authors and Affiliations
Contributions
HT designed the study, undertook most of the experimental work, and drafted the manuscript. XY and XC undertook part of the experimental work. HD and XH provide support for chemical analysis and testing. CL and CX contributed substantially to the revising of this work. FZ acquired the funding used to support this work, and provided substantial support of the design, drafting and revision of this work.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, H., Yan, X., Zhou, F. et al. Effect of process conditions on generation of hydrochloric acid and lithium hydroxide from simulated lithium chloride solution using bipolar membrane electrodialysis. SN Appl. Sci. 4, 47 (2022). https://doi.org/10.1007/s42452-021-04914-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04914-9