Abstract
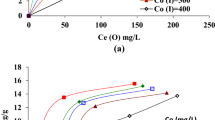
Comparisons of protocatechuic acid (PA) and caffeic acid (CA) adsorption isotherm on C18 column and competitive adsorption of the two compounds were investigated. By linear and nonlinear regression analysis, the experimental parameters in the equilibrium isotherms were estimated. Adsorption equilibrium data of the two compounds were investigated using six different models including linear, Langmuir, Freundlich, Langmuir-Freundlich, competitive Langmuir and Quadratic. In the moderate range of concentrations, the competitive Langmuir isotherm proved to be the best model for these experimental data. The regression coefficients of the competitive Langmuir adsorption isotherms were 0.9860 for PA and 0.9898 for CA, respectively. The coefficients obtained for the six isotherm models confirmed the superiority of the competitive Langmuir isotherm for analyzing the competitive adsorption data of solutes.
Similar content being viewed by others
References
Y. Z. Jin and K. H. Row, Korean J. Chem. Eng., 22(2), 264 (2005).
G. Georges, J. Chromatogr. A, 965, 129 (2002).
G. Fabrice and G. Georges, J. Chromatogr. A, 1008, 23 (2003).
M. Juza, J. Chromatogr. A, 865, 35 (1999).
E. Huthmann and M. Juza, J. Chromatogr. A, 908, 185 (2001).
S. Khattabi, D. E. Cherrak and K. Mihlbachler, J. Chromatogr. A, 893, 307 (2000).
Y. J. Yang, C. H. Lee and Y.M. Koo, Biotechnol. Bioprocess Eng., 9, 331 (2004).
C. Heuer, E. Kusters, T. Plattner and A. Seidel-Morgenstern, J. Chromatogr. A, 827, 175 (1998).
K. H. Row, C. H. Lee and J. H. Kang, Biotechnol. Bioprocess Eng., 7, 11 (2002).
S. Toda, T. Miyase, H. Arichi, H. Tanizawa and Y. Takano, Chem. Pharm. Bull., 33, 1725 (1985).
T. Tanaka, T. Kawamori, M. Ohnishi, K. Okamoto, H. Mori and A. Hara, Cancer Res., 54, 2359 (1994).
T. Tanaka, T. Kojima, T. Kawamori, Y. Hirose, M. Ohnishi and H. Mori, Cancer Res., 54, 116 (1994).
T. Tanaka, T. Kojima, M. Suzui and H. Mori, Cancer Res., 53, 3908 (1993).
T. Tanaka, T. Kojima, T. Kawamori, N. Yoshimi and H. Mori, Cancer Res., 53, 9775 (1993).
Y. Bayrak, Micropor. Mesopor. Mater., 87, 203 (2006).
F. Gritti and G. Guiochon, J. Chromatogr. A, 1115, 142 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, C.H., Zhu, T. & Row, K.H. Competitive adsorption of protocatechuic acid and caffeic acid on C18 particles. Korean J. Chem. Eng. 29, 135–138 (2012). https://doi.org/10.1007/s11814-011-0152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0152-z