Abstract
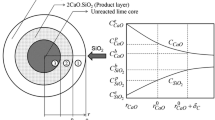
The rate at which limestone dissolves is very important in wet flue gas desulfurization process (FGD). High dissolution rates provide better alkalinity, which is important for sulfur dioxide (SO2) absorption. This study investigates the use of urea to improve the dissolution rate of limestone. The dissolution characteristics have been studied by using a pH-Stat method. The dissolution rate constant was measured according to the shrinking core model with surface control, i.e. (1−(1−X)1/3)=k r t. The effect of experimental variables such as temperature, amount of urea, solid to liquid ratio and stirring speed on the dissolution rate of limestone were investigated. Using a central composite design (CCD) of experiments variables, a mathematical model was developed to correlate the experimental variables to the dissolution rate constant. The experimental value was found to agree satisfactorily with predicted dissolution rate constant. The model shows that high temperature and low solid to liquid ratio improves the dissolution rate. The dissolution rate increased slightly with increase in the stirring speed. In the presence of urea the dissolution rate constant increased by 122%. The dissolution reaction follows a shrinking-core model with the chemical reaction control as the rate-controlling step.
Similar content being viewed by others
References
S. F. Randall and D. K. Matibe, Energy Policy, 31, 721 (2003).
J. Kaminski, Appl. Energy, 75, 165 (2003).
H. K. Lee, B.R. Deshwal and K. S. Yoo, Korean J. Chem. Eng., 22, 208 (2005).
M. Kang, J. H. Park, J. S. Choi, E. D. Park and J. E. Yie, Korean J. Chem. Eng., 24, 191 (2007).
J. H. Choi, J. H. Kim, Y. C. Bak, R. Amal and J. Scott, Korean J. Chem. Eng., 22, 844 (2005).
S. Uchida, H. Moriguchi, H. Maejima, K. Koide and S. Kageyama, Canadian J. Chem. Eng., 56, 690 (1978).
L. Eisenlohr, K. Meteva, F. Gabrovsek and W. Dreybrodt, Geochimet Coschimica Acta, 63, 989 (1999).
L. Plan, Geomopho., 68, 201 (2005).
Z.O. Siagi and M. M. Mbarawa, J. Hazard. Mater., 163, 678 (2007).
S.M. Shih, J. P. Lin and G.Y. Shiau, J. Hazard. Mater., 79, 159 (2000).
C. Hosten and M. Gulsun, Min. Eng., 17, 97 (2004).
G. T. Hefter and R. P. T. Tomkins, The experimental determination of solubilities, John Wiley (2003).
A. Stergarsek M. Gerbec, R. Kocjančič and P. Frkal, Acta Chim. Slov., 46, 323 (1999).
T. Takashina, S. Honjo, N. Ukawa and K. Iwashita, Soc. Chem. Eng. Japan., 35, 197 (2002).
H. L. Rutto, Z.O. Ziagi and M.M. Mbarawa, J. Hazard. Mater., 168, 1532 (2009).
K. Sekiguchi and N. Obi, Man. Chem. Pharm. Bull., 9, 866 (1961).
S. Hausmanns, G. Laufenberg and B. Kunz, Desalination, 104 95(1996).
D. C. Montgomery, Design and analysis of experiments, John Wiley and Sons Ltd., New York (2001).
D. C. Drehmel, Symp., 46, 123 (2001).
J. Ahlbeck, T. Engman and M. Vihma, Chem. Eng. Sci., 48, 3479 (1993).
J. Ahlbeck, T. Engman and M. Vihma, Chem. Eng. Sci., 50, 1081 (1995).
P.V. Danckwerts, Gas-liquid reactions, McGraw-Hill, New York (1970).
O. Levenspiel, Chemical reaction engineering, John Wiley and Sons, New York (1972).
X. Gao, R. Guo, H. Ding, Zh. Luo and K. Cen, J. Hazard. Mater., 168, 1059 (2009).
A. Aydogan, M. Erdemoglu and G. Ucar, Hydrometa., 88, 52 (2007).
P. K. Calderbank and M. B. Moo-Young, Chem. Eng. Sci., 16, 39 (1961).
P. Harriott, AIChE J., 8, 93 (1962).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutto, H., Enweremadu, C. Dissolution of a South African calcium based material using urea: An optimized process. Korean J. Chem. Eng. 29, 1–8 (2012). https://doi.org/10.1007/s11814-011-0136-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0136-z