Abstract
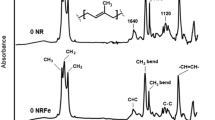
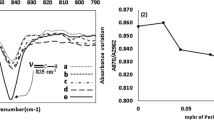
Natural rubber (NR) can be degraded depending on various factors such as heat, mechanical force, chemical reaction, and light. Light is a very interesting factor because it can cause the NR to degrade under low temperature and pressure. The photo-degradation of NR films was carried out to investigate the effects of the light and the temperature on the reduction of the weight-average molecular weight (Mw) and the double bonds in the NR films. The NR films, with and without catalysts, titanium dioxide (TiO2), and potassium persulfate (K2S2O8), were exposed to light from a mercury light bulb at 7,000 and 36,000 lux, and at the temperature of 25 °C and 80 °C for 192 hrs. After exposure, the Mw of the NR films was analyzed by gel permeation chromatography (GPC). Changes in the Mw were used to construct a kinetic model for the process, (1/Mw)=(1/Mw0)+(kt/2M0) where k is the rate constant, and M0 is the Mw of the monomer unit. The linear relationship between 1/Mw and time suggested pseudo first-order processes with random scission. The Mw distribution information from the GPC was used to calculate the number of double bonds in the NR films. The trend of the double bonds reduction curves was quite similar to the result obtained from the calculation from the FTIR spectra. This indicated that this calculation method might possibly be another alternative way to obtain the number of double bonds in the NR.
Similar content being viewed by others
References
T. Kelen, Polymer degradation, Van Nostrand Reinhold, New York (1983).
C. Adam, J. Lacoste and J. Lemaire, Polym. Degrad. Stab., 32, 51 (1991).
R. N. Christopher and D. Forciniti, Indus. Eng. Chem. Res., 40, 3346 (2001).
A. Copinet, C. Bertran, S. Govindin, V. Coma and Y. Couturier, Chemosphere, 55, 763 (2004).
C. Naddeo, L. Guadagno and V. Vittoria, Polym. Degrad. Stab., 85, 1009 (2004).
K.A. M. Santos, P.A. Z. Suarez and J.C. Rubim, Polym. Degrad. Stab., 90, 34 (2005).
J. Gijsman, G. Meijers and G. Vitae, Polym. Degrad. Stab., 65, 433 (1999).
C. M. Maillo and J.R. White, Plast. Rubb. Comp., 28, 277 (1999).
A. L. Andrady, S.H. Hamid, X. Hu and A. Torikai, J. Photochem. Photobio. B., 46, 96 (1998).
I.M. Arabatzis, T. Stergiopoulos, M. C. Bernard, D. Labou, S.G. Neophytides and P. Falaras, Appl. Cat. B., 42, 187 (2003).
T. Zhang, T. Oyama, S. Horikoshi, J. Zhao, N. Serpone and H. Hidaka, Appl. Cat. B., 42, 13 (2003).
L. Zhang, Y. Zhu, Y. He, W. Li and H. Sun, Appl. Cat. B., 40, 287 (2003).
V.A. Sakkas, I. M. Arabatzis, I.K. Konstantinou, A.D. Dimou, T.A. Albanis and P. Falaras, Appl. Cat. B., 49, 195 (2004).
I.K. Kim, H. J. Ha and S. K. Lee, Korean J. Chem. Eng., 22, 382 (2005).
S. J. Yoa, Y. S. Cho and J.H. Kim, Korean J. Chem. Eng., 22, 364 (2005).
R. Thiruvenkatachari, T. O. Kwon and I. S. Moon, Korean J. Chem. Eng., 22, 938 (2005).
K. N. Kim and M.R. Hoffmann, Korean J. Chem. Eng., 25, 89 (2008).
D. R. Park, B. J. Ahn, H. S. Park, H. Yamashita and M. Anpo, Korean J. Chem. Eng., 18, 930 (2001).
S. Cho and W. Choi, J. Photochem. Photobio. A., 143, 221 (2001).
J. Shang, M. Chai and Y. Zhu, J. Solid. State. Chem., 174, 104 (2003).
J. Shang, M. Chai and Y. Zhu, Envi. Sci. Tech., 37, 4493 (2003).
H. Kubota, Y. Hariya, S. Kuroda and T. Kondo, Polym. Degrad. Stab., 72, 223 (2001).
J. P. Roubroeks, R. Andersson, D. I. Mastromauro, B. E. Christensen and P. Aman, Carbo. Polm., 46, 275 (2001).
C. Tanford, Physical chemistry of macromolecules, Wiley, New York (1961).
H.R. Allcock, F.W. Lampe and J. E. Mark, Contemporary polymer chemistry, Pearson Education, New Jersey (2003).
T. J. Turton and J. R. White, Polym. Degrad. Stab., 74, 559 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tasakorn, P., Amatyakul, W. Photochemical reduction of molecular weight and number of double bonds in natural rubber film. Korean J. Chem. Eng. 25, 1532–1538 (2008). https://doi.org/10.1007/s11814-008-0252-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-008-0252-6