Abstract
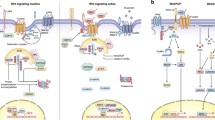
Wnt signaling has been shown to engage a multifunctional pathway that is involved in the regulation of a wide variety of normal and pathologic processes, including embryogenesis, differentiation and tumorigenesis. Recent studies have demonstrated that Wnt5a expression is frequently seen in various human cancers. In contrast to the transforming members of the Wnt family, shown to be upregulated in many cancers, the role of Wnt5a is still controversial in its expression in different tumors. There is increasing evidence that Wnt5a has tumor suppressor function in some malignancies, and in addition, it elicits promigratory and proinvasive effects via the planar cell polarity pathway, which suggests that Wnt5a might be an effective marker for the progression and prognosis of tumors. Obviously, the outcome of Wnt5a signaling is dependent on a multitude of variables, ranging from receptors, downstream effectors and inhibitors, to external influences coming from the tumor microenvironment. This review will focus on the role of Wnt5a signaling and, as a consequence, provide an outline describing the expression and functions of Wnt5a in cancer progression.
Similar content being viewed by others
References
Clevers H. Wnt/β-catenin signaling in development and disease. Cell 2006; 127: 469–480.
Nusse R. Wnt signaling in disease and development. Cell Res 2005; 15: 28–32.
Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol 1994; 14: 6278–6286.
Olson DJ, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ 1994; 5: 197–206.
Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998; 14: 59–88.
Kuhl M, Sheldahl LC, Park M, et al. The Wnt/Ca2+ pathway: a new vertebrate wnt signaling pathway takes shape. Trends Genet 2000; 16: 279–283.
Bhanot P, Brink M, Samos CH, et al. A new member of the frizzled family from Drosophila functions as a wingless receptor. Nature 1996; 282: 225–230.
Yang-Snyder J, Miller JR, Brown JD, et al. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol 1996; 6: 1302–1306.
Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol 1998; 8: 591–594.
Kishida S, Yamamoto H, Hino S, et al. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol 1999; 19: 4414–4422.
Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem 1999; 274: 21464–21470.
Rubinfeld B, Albert I, Porfiri E, et al. Binding of GSK3-β to the APC-β-catenin complex and regulation of complex assembly. Science 1996; 272: 1023–1026.
Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382: 638–642.
Sakanaka C, Leong P, Xu L, et al. Casein kinase-1 epsilon in the wnt pathway: Regulation of beta-catenin function. Proc Natl Acad Sci 1999; 96: 12548–12552.
Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem 1999; 274: 3439–3445.
Sheldahl LC, Slusarski DC, Pandur P, et al. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 2003; 26: 769–777.
Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 2005; 132: 4421–4436.
Seifert JR, Mlodzik M. Frizzled/PCP signaling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 2007; 8: 126–138.
Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays 2007; 29: 120–132.
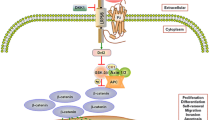
Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in noncanonical Wnt5a/JNK signaling pathway. Genes Cells 2003; 8: 645–654.
Schambony A, Wedlich D. Wnt-5a/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 2007; 5: 779–792.
Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits b-catenin-TCF signaling depending on receptor context. PLoS Biol 2006; 4: 570–582.
Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 2005; 437: 281–285.
He X, Saint-Jeannet JP, Wang Y, et al. A member of the frizzled protein family mediating axis induction by Wnt 5a. Science 1997; 275: 1652–1654.
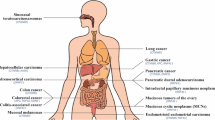
Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt-5a in human malignancy 1995; l16: 3299–3306.
Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000; 406: 536–540.
Weeraratna AT, Jiang Y, Hostetter G, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1: 279–288.
O’Connell MP, French AD, Leotlela PD, et al. Assaying Wnt5a-mediated invasion in melanoma cells. Methods Mol Biol 2008; 468: 243–53.
Bachmann IM, Straume O, Puntervoll HE, et a1. Importance of P-cadherin, beta-catenin, and Wnt-5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res 2005; 11(24 Pt 1).
Kurayoshi M, Oue N, Yamamoto H, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66:10439–10448.
Liu XH, Zhou HB, Ma HH,et al. Expression of Wnt5a, β-catenin and E-cadherin in hepatocellular carcinoma and its clinical significance. Chin J Clin Exp Pathol 2007; 23: 400–403 (Chinese).
Ripka S, Konig A, Buchholz M, et al. WNT5A-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 2007; 6: 1178–1187.
Dejmek J, Dejmek A, Safholm A, et al. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 2005; 65: 9142–9146.
Smith K, Bui TD, Poulsom R, et al. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer 1999; 81: 496–5021.
Lejeune S, Hugnet EL, Hamby A, et a1. Wnt-5a cloning, expression, and up regulation in human primary breast cancers. Clin Cancer Res 1995; 1: 215–222.
Jönsson M, Dejmek J, Bendahl PO, et al. Loss of Wnt- 5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 2002; 62: 409–416.
Kremenevskaja N, von Wasielewski R, Rao AS, et al. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 2005; 24: 2144–2154.
Bui TD, Zhang L, Rees MC, et al. Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer 1997; 75: 1131–1136.
Blanc E, Goldschneider D, Douc-Rasy S, et al. Wnt- 5a gene expression in malignant human neuroblasts. Cancer Lett 2005; 228: 117–123.
Liang H, Chen Q, Coles AH, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoetic tissue. Cancer Cell 2003; 4: 349–360.
Zeng ZY, Zhou YH, Zhang WL, et al. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol 2007; 38: 120–133.
Jönsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci 2001; 114: 2043–2053.
Tulac S, Nayak NR, Kao LC, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 2003; 88: 3860–3866.
Dejmek J, Dejmek A, Safholm A, et al. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 2005; 65: 9142–9146.
Säfholm A, Leandersson K, Dejmek J, et al. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breastepithelial cells. J Biol Chem 2006; 281: 2740–49.
Nishita M, Yoo SK, Nomachi A, et al. Flopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 2006; 175: 555–562.
Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 2005; 129: 626–638.
Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 2003; 13: 366–375.
Mareel M, Leroy A. Clinical, cellular and molecular aspects of cancer invasion. Physiol Rev 2003; 83: 337–376.
Blumenthal A, Ehlers S, Lauber J, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006; 108: 965–973.
Pukrop T, Klemm F, Hagemann T, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA 2006; 103: 5454–5459.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shen, Xh., Li, D., Li, Hy. et al. Wnt5a signaling — A new and attractive target for specific anticancer therapy. Clin. Oncol. Cancer Res. 7, 1–6 (2010). https://doi.org/10.1007/s11805-010-0001-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11805-010-0001-6