Abstract
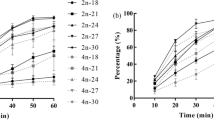
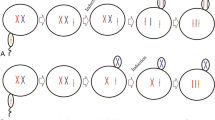
In order to evaluate the effects of triploidy induction on a selected strain ‘Haida No. 2’ of the Pacific oyster Crassostrea gigas, which is characterized with golden shell color and high growth rate, the growth, survival rate and stability of triploid rate were analyzed at different development stages in the present study. Three different conditions inhibiting the release of polar body II or polar body I were tested: (A) Cytochalasin-B (CB), 0.5 mg L−1 at 10 min post-insemination for 15 min; (B) CB, 0.5 mg L−1at 15 min post-insemination for 20 min; and (C) CB, 0.7 mg L−1, at 15 min post-insemination for 20 min. The triploidy induction treatments significantly reduced the D-larvae and survival rates at the larvae stage but not at the juvenile and adult stages. Triploid rate dramatically decreased at the larval stage and did not significantly change at the juvenile and adult stages. Regarding the stability of the triploid rate, there was a significant difference between the three treatment groups. Larvae from the treatment A and control groups exhibited higher growth rates in shell height than those from the other two treatment groups at day 27. Triploid juveniles and adults from the treatment A group exhibited a higher wet weight than diploids from the control group and triploids from the other treatment groups. Triploidy induction did not affect the shell color of the progeny. The results obtained in the study demonstrate that triploidy induction has the potential to be used to increase the production of C. gigas variety ‘Haida No. 2’ without modifying its golden shell color.
Similar content being viewed by others
References
Allen Jr., S. K., and Downing, S. L., 1986. Performance of triploid Pacific oysters, Crassostrea gigas (Thunberg). I. Survival, growth, glycogen content, and sexual maturation in yearlings. Journal of Experimental Marine Biology and Ecology, 102: 197–208.
Benfey, T. J., 1999. The physiology and behavior of triploid fishes. Reviews in Fisheries Science, 7: 39–67
Buestel, D., Ropert, M., Prou, J., and Goulletquer, P., 2009. History, status, and future of oyster culture in France. Journal of Shellfish Research, 28: 813–820.
Callam, B. R., Allen Jr., S. K., and Frank-Lawale, A., 2016. Genetic and environmental influence on triploid Crassostrea virginica grown in Chesapeake Bay: Growth. Aquaculture, 452: 97–106.
Cheney, D. P., 2000. Summer mortality of Pacific oysters, Crassostrea gigas (Thunberg): Initial finding on multiple environmental stressors in Puget Sound, Washington, 1998. Journal of Shellfish Research, 19: 353–359.
Desrosiers, R. R., Gérard, A., Peignon, J. M., Naciri, Y., Dufresne, L., Morasse, J., et al., 1993. A novel method to produce triploids in bivalve molluscs by the use of 6-dimethylaminopurine. Journal of Experimental Marine Biology and Ecology, 170: 29–43.
Garnier-Géré, P. H., Naciri-Graven, Y., Bougrier, S., Magoulas, A., Héral, M., Kotoulas, G., et al., 2002. Influences of triploidy, parentage and genetic diversity on growth of the Pacific oyster Crassostrea gigas reared in contrasting natural environments. Molecular Ecology, 11: 1499–1514.
Ge, J. L., 2015. Selective breeding and genetic analysis of shell color traits in the Pacific oyster, Crassostrea gigas. PhD thesis. Ocean University of China (in Chinese with English abstract).
Gérard, A., Ledu, C., Phélipot, P., and Naciri-Graven, Y., 1999. The induction of MI and MII triploids in the Pacific oyster Crassostrea gigas with 6-DMAP or CB. Aquaculture, 174: 229–242.
Gerard, A., Naciri, Y., Peignon, J. M., Ledu, C., and Phelipot, P., 1994. Optimization of triploid induction by the use of 6-DMAP for the oyster Crassostrea gigas (Thunberg). Aquaculture Research, 25: 709–719.
Guo, X. M., and Allen Jr., S. K., 1994. Reproductive potential and genetics of triploid Pacific oysters, Crassostrea gigas (Thunberg). The Biological Bulletin, 187: 309–318.
Guo, X. M., Cooper, K., Hershberger, W. K., and Chew, K. K., 1992. Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific oyster, Crassostrea gigas: I. Ploidy of resultant embryos. The Biological Bulletin, 183: 381–386.
Hawkins, A. J. S., Day, A. J., Gérard, A., Naciri, C., Ledu, C., Bayne, B. L., et al., 1994. A genetic and metabolic basis for faster growth among triploids induced by blocking meiosis I but not meiosis II in the larviparous European flat oyster, Ostrea edulis L. Journal of Experimental Marine Biology and Ecology, 184: 21–40.
Houssin, M., Trancart, S., Denechere, L., Oden, E., Adeline, B., Lepoitevin, M., et al., 2019. Abnormal mortality of triploid adult Pacific oysters: Is there a correlation with high gametogenesis in Normandy, France?. Aquaculture, 505: 63–71.
Jeung, H. D., Keshavmurthy, S., Lim, H. J., Kim, S. K., and Choi, K. S., 2016. Quantification of reproductive effort of the triploid Pacific oyster, Crassostrea gigas raised in intertidal rack and bag oyster culture system off the west coast of Korea during spawning season. Aquaculture, 464: 374–380.
Kong, L. F., Song, S. L., and Li, Q., 2017. The effect of interstrain hybridization on the production performance in the Pacific oyster Crassostrea gigas. Aquaculture, 472: 44–49.
Li, Q., Wang, Q. Z., Liu, S. K., and Kong, L. F., 2011. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fisheries Science, 77: 643–648.
Melo, E. M. C., Gomes, C. H. A. M., Silva, F. C. D., Sühnel, S., and Melo, C. M. R. D., 2015. Chemical and physical methods of triploidy induction in Crassostrea gigas (Thunberg, 1793). Boletim do Instituto de Pesca, 414: 889–898.
Nell, J. A., 2001. The history of oyster farming in Australia. Marine Fisheries Review, 63: 14–25.
Nell, J. A., 2002. Farming triploid oysters. Aquaculture, 210: 69–88.
Nell, J. A., and Perkins, B., 2005. Studies on triploid oysters in Australia: Farming potential of all-triploid Pacific oysters, Crassostrea gigas (Thunberg), in Port Stephens, New South Wales, Australia. Aquaculture Research, 36: 530–536.
Qin, Y. P., Zhang, Y. H., Ma, H. T., Wu, X. W., Xiao, S., Li, J., et al., 2018. Comparison of the biochemical composition and nutritional quality between diploid and triploid Hong Kong oysters, Crassostrea hongkongensis. Frontiers in physiology, 9: 1674.
Qin, Y. P., Zhang, Y. H., Mo, R. G., Zhang, Y., Li, J., Zhou, Y. L., et al., 2019. Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oysters Crassostrea hongkongensis in southern China. Aquaculture, 507: 108–118.
Smith, I. R., Nell, J. A., and Adlard, R., 2000. The effect of growing level and growing method on winter mortality, Mikrocytos roughleyi, in diploid and triploid Sydney rock oysters, Saccostrea glomerata. Aquaculture, 185: 197–205.
Stanley, J. G., Hidu, H., and Allen Jr., S. K., 1984. Growth of American oysters increased by polyploidy induced by blocking meiosis I but not meiosis II. Aquaculture, 37: 147–155.
Supan, J. E., Wilson, C. E., and Allen Jr., S. K., 2000. The effect of cytochalasin B dosage on the survival and ploidy of Crassostrea virginica (Gmelin) larvae. Journal of Shellfish Research, 19: 125–128.
Toro, J. E., Sanhueza, M. A., Paredes, L., and Canello, F., 1995. Induction of triploid embryos by heat shock in the Chilean northern scallop Argopecten purpuratus Lamarck, 1819. New Zealand Journal of Marine and Freshwater Research, 29: 101–105.
Wadsworth, P., Wilson, A. E., and Walton, W. C., 2019. A meta-analysis of growth rate in diploid and triploid oysters. Aquaculture, 499: 9–16.
Wang, Z. P., Guo, X. M., Allen Jr., S. K., and Wang, R. C., 2002. Heterozygosity and body size in triploid Pacific oysters, Crassostrea gigas Thunberg, produced from meiosis II inhibition and tetraploids. Aquaculture, 204: 337–348.
Yang, H. P., and Guo, X. M., 2006. Polyploid induction by heat shock-induced meiosis and mitosis inhibition in the dwarf surfclam, Mulinia lateralis Say. Aquaculture, 252: 171–182.
Zouros, E., Romero-Dorey, M., and Mallet, A. L., 1988. Heterozygosity and growth in marine bivalves: Further data and possible explanations. Evolution, 42: 1332–1341.
Acknowledgements
This work was supported by the grants from the China Agriculture Research System Project (No. CARS-49), and the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (Nos. 2020LZGC016, 2021 LZGC027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Li, Q., Liu, Y. et al. Productive Traits and Triploid Rate Stability in Triploidy-Induced ‘Haida No. 2’ Strain of the Pacific Oyster, Crassostrea gigas. J. Ocean Univ. China 22, 229–234 (2023). https://doi.org/10.1007/s11802-023-5183-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-023-5183-7