Abstract
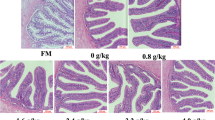
Soybean stachyose (SBS) and phytic acid (PA) are anti-nutritional factors (ANF) which have deleterious effects on the growth and digestibility in fish. The present research studied the effects of dietary SBS and PA on the expression of three serine protease genes in the liver of Japanese flounder (Paralichthys olivaceus). These genes are trypsinogen 1 (poTRY), elastase 1 (poEL) and chymotrypsinogen 1 (poCTRY). Eight artificial diets with graded levels of supplemented ANFs were formulated to 4 levels of SBS (0.00, 0.40, 0.80 and 1.50%), 4 levels of PA (0.00, 0.20, 0.40 and 0.80), respectively. Japanese flounder (initial weight 2.45 g ± 0.01 g) were fed with these diets for 10 weeks with three replications per treatment. At the end of 10 weeks, supplementation of 0.40% of dietary SBS or PA significantly increased the gene expression of poTRY and poCTRY (P<0.05). The same level of dietary SBS significantly decreased the gene expression of poEL. In comparison with the control group (ANF-free), dietary PA (0.2% and 0.8%) significantly decreased the gene expression of poTRY, poCTRY and poEL (P<0.05). However, excessive supplement of dietary SBS (1.5%) has no significant effects on these gene expressions (P>0.05). These results suggested that dietary SBS and dietary PA could directly affect the serine protease genes at the transcriptional level in Japanese flounder, and these genes’ expression was more sensitive to dietary PA than to SBS under the current experimental conditions.
Similar content being viewed by others
References
Alarcon, F. J., Garcia-Carreno, F. L., Navarrete, and Del Toro M. A., 2001. Effect of plant protease inhibitors on digestive proteases in two fish species, Lutjanus argentiventris and L. novemfasciatus. Fish Physiology and Biochemistry, 24: 179–189.
Bakke-McKellep, A. M., Penn, M. H., Salas, P. M., Refstie, S., Sperstad, S., Landsverk, T., Ringo, E., and Krogdahl, A., 2007. Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.). British Journal of Nutrition, 97: 699–713.
Barrett, A. J., Rawlings, N. D., and Woessner, J. F., 2004. Serine and threonine peptidases. In: Handbooks of Proteolytic Enzymes, Vol. II. Barrett, A. J., et al., eds., Elsevier Academic Press, London, 1417–1447.
Bohn, T., Davidsson, L., Walczyk, T., and Hurrell, R. F., 2004. Phytic acid added to white-wheat bread inhibits fractional apparent magnesium absorption in humans. The American Journal of Clinical Nutrition, 79: 418–423.
Bradford, M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248–254.
Burel, C., Boujard, T., Escaffre, A. M., Kaushik, S. J., Boeuf, G., Mol, K. A., Van der Geyten, S., and Kühn, E. R., 2000. Dietary low-glucosinolate rapeseed meal affects thyroid status and nutrient utilization in rainbow trout (Oncorhynchus mykiss). British Journal of Nutrition, 83(6): 653–664.
Cai, C. F., Wang, W. J., Ye, Y. T., Krogdahl, A., Wang, Y. L., Xia, Y. M., and Yang, C. G., 2011. Effect of soybean meal, raffinose and stachyose on the growth, body composition, intestinal morphology and intestinal microflora of juvenile allogynogenetic silver crucian carp (Carassius auratus gibelio♀ × Cyprinus carpio♂). Aquaculture Research, doi:10.1111/j.-1365-2109.2011.02811.x.
Dabrowski, K., Poczyczynski, P., and Berger, R., 1989. Effect of partially or totally replacing fishmeal protein by soybean meal protein on growth, food utilisation and proteolytic enzyme activities in rainbow trout (Salmo gairdneri). New in vivo test for endocrine pancreatic secretion. Aquaculture, 77: 29–49.
Deng, J. M., Mai, K. S., Ai, Q. H., and Zhang, W. B., 2007. Effects of soybean oligosaccharides on lipid metabolism of Japanese flounder (Paralichthys olivaceus Temminck et Schlegel) fed animal or plant protein source-based diets. Frontiers of Agriculture in China, 1(3): 1–9 (in Chinese with English abstract).
Deng, J. M., Mai, K. S., Ai, Q. H., and Zhang, W. B., 2009a. Effects of soybean oligosaccharides on nutritional characters of Japanese Flounder (Paralichthys olivaceus): I. Feeding rate, growth and metabolize enzyme activities. Acta Hydrobiologica Sinica, 33(1): 7–14 (in Chinese with English abstract).
Deng, J. M., Mai, K. S., Ai, Q. H., and Zhang, W. B., 2009b. Effects of soybean oligosaccharides on nutritional characters of Japanese Flounder (Paralichthys olivaceus): II. Digestibility and digestive physiology. Acta. Hydrobiologica Sinica, 33(3): 369–375 (in Chinese with English abstract).
Glencross, B. D., Boujard, T., and Kaushik, S. J., 2003. Influence of oligosaccharides on the digestibility of lupin meals when fed to rainbow trout, Oncorhynchus mykiss. Aquaculture, 219: 703–713.
Gomes, E. F., Rema, P., and Kaushik, S. J., 1995. Replacement of fish meal by plant proteins in the diets of rainbow trout (Oncorhynchus mykiss): digestibility and growth performance. Aquaculture, 130: 177–186.
Grisdale-Helland, B., Helland, S. J., and Gatlin III, D. M., 2008. The effects of dietary supplementation with mannanoligo-saccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture, 283: 163–167.
Guttieri, M. J., Peterson, K. M., and Souza, E. J., 2006. Milling and baking quality of low phytic acid wheat. Crop Science, 46: 2403–2408.
Heikkinen, J., Vielma, J., Kemilainen, O., Tiirola, M., Eskelinen, P., Kiuru, T., Navia-Paldanius, D., and von Wright, A., 2006. Effects of soybean meal based diets on growth performance, gut histopathology and intestinal microbiota of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture, 261: 259–268.
Isnard, N., Péterszegi, G., Robert, A. M., and Robert, L., 2002. Regulation of elastase-type endopeptidase activity, MMP-2 and MMP-9 expression and activation in human dermal fibroblasts by fucose and a fucose-rich polysaccharide. Biomedicine and Pharmacotherapy, 56(5): 258–264.
Kaushik, S. J., Cravedi, J. P., and Lalles, J. P., 1995. Partial or total replacement of fish meal by soyabean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quantity in rainbow trout, Oncorhynchus mykiss. Aquaculture, 133: 257–274.
Kikuchi, K., 1999. Partial replacement of fish meal with corn gluten meal in diets for Japanese flounder Paralichthys olivaceus. The Journal of the World Aquaculture Society, 30: 357–363.
Konishi, C., Matsui, T., Park, W., Yano, H., and Yano, F., 1999. Heat treatment of soybean meal and rapeseed meal suppresses rumen degradation of phytate phosphorus in sheep. Animal Feed Science and Technology, 80: 115–122.
Krogdahl, A., Hemre, G.-I., and Mommsen, T. P., 2005. Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquaculture Nutrition, 111: 103–122.
Leske, K. L., Jevne, C. J., and Coon, C. N., 1993. Effect of oligosaccharide additions on nitrogen-corrected true metabolizable energy of soy protein concentrate. Poultry Science, 72(4): 664–668.
Liener, I. E., 1995. Possible adverse effects of soybean anticarcinogens. Journal of Nutrition, 125(3 Suppl): 744S–750S.
Lilleeng, E., Froystad, M. K., Ostby, G. C., Valen, E. C., and Krogdahl, A., 2007. Effects of diets containing soybean meal on trypsin mRNA expression and activity in Atlantic salmon (Salmo salar L.). Comparative Biochemistry and Physiology (A), 147: 25–36.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25: 402–408.
Mente, E., Deguara, S., Santos, M. B., and Houlihan, D., 2003. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (Salmo salar L.). Aquaculture, 225: 133–147.
Namkung, H., and Leeson, S., 1999. Effect of phytase enzyme on dietary nitrogen-corrected apparent metabolizable energy and the ileal digestibility of nitrogen and amino acids in broiler chicks. Poultry Science, 78(9): 1317–1319.
Olli, J. J., Krogdahl, Å., and Våbenø, A., 1995. Dehulled solvent-extracted soybean meal as a protein source in diets for Atlantic salmon, Salmo salar L. Aquaculture Research, 26: 167–174.
Refstie, S., Landsverk, T., Bakke-McKellep, A. M., Ringø, E., Sundby, A., Shearer, K. D., and Krogdahl, Å., 2006. Digestive capacity, intestinal morphology, and microflora of 1-year and 2-year old Atlantic cod (Gadus morhua) fed standard or bioprocessed soybean meal. Aquaculture, 261: 269–284.
Refstie, S., Sahlström, S., Bråthen, E., Baeverfjord, G., and Krogedal, P., 2005. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture, 246: 331–345.
Refstie, S., Storebakken, T., and Roem, A. J., 1998. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture, 162: 301–312.
Refstie, S., Storebakken, T., Baeverfjord, G., and Roem, A. J., 2001. Long-term protein and lipid growth of Atlantic salmon Salmo Salar fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level. Aquaculture, 193: 91–106.
Robaina, M. S., Izquierdo, F., and Moyanob, J., 1995. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture, 130: 219–233.
Singh, M., and Krikorian, A. D., 1982. Inhibition of trypsin activity in vitro by phytate. Journal of Agricultural and Food Chemistry, 30: 799–800.
Smits, C. H. M., and Annison, G., 1996. Non-starch plant polysaccharides in broiler nutrition-towards a physiologically valid approach to their determination. World’s Poultry Science Journal, 52: 204–221.
Sørensen, M., Penn, M., El-Mowafi, A., Storebakken, T., Cai, C., èverland, M., and Krogdahl, Å., 2011. Effect of stachyose, raffinose and soya-saponins supplementation on nutrient digestibility, digestive enzymes, gut morphology and growth performance in Atlantic salmon (Salmo salar, L). Aqua-culture, doi:10.1016/j.aquaculture.2011.02.013
Stein, H. H., Trottier, N. L., Bellaver, C., and Easter, R. A., 1999. The effect of feeding level and physiological status on total flow and amino acid composition of endogenous protein at the distal ileum in swine. Journal of Animal Science, 77(5): 1180–1187.
Storebakken, T., Kvien, I. S., Shearer, K. D., Grisdale-Helland, B., Helland, S. J., and Berge, G. M., 1998. The apparent digestibility of diets containing fish meal, soybean meal or bacterial meal fed to Atlantic salmon (Salmo salar): evaluation of different faecal collection methods. Aquaculture, 169: 195–210.
Uran, P. A., Schrama, J. W., Jaafari, S., Baardsen, G., Rombout, J. H. W. M., and Verreth, J. A. J., 2008. Variation in commercial sources of soybean meal influences the severity of enteritis in Atlantic salmon (Salmo salar L.). Aquaculture Nutrition, 15: 492–499.
Vielma, J., Makinen, T., and Ekholm, P., 2000. Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout Oncorhynchus mykiss and algal availability of phosphorus load. Aquaculture, 183: 349–362.
Vila, B., and Mascarell, J., 1999. α-Galactosides in soybean meal: can enzyme help? Feed International, 6: 24–29.
Weeks, C., Garling, D., Barrows, F. T., and Faisal, M., 2010. The effect of feeding varying levels of soybean meal in high-nutrient-density diets on growth performance and body composition of juvenile Atlantic salmon. North American Journal of Aquaculture, 72(4): 279–289.
Yigit, M., Koshio, S., Teshima, S., and Ishikawa, M., 2004. Dietary protein and energy requirements of juvenile Japanese flounder, Paralichthys olivaceus. Journal of Applied Sciences, 4: 486–492.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mi, H., Mai, K., Zhang, W. et al. Effects of dietary soybean stachyose and phytic acid on gene expressions of serine proteases in Japanese flounder (Paralichthys olivaceus). J. Ocean Univ. China 10, 234–240 (2011). https://doi.org/10.1007/s11802-011-1853-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-011-1853-y