Abstract
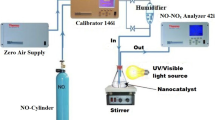
This work describes the environmentally friendly technology for oxidation of ammonia (NH3) to form nitrogen at temperatures range from 423K to 673K by selective catalytic oxidation (SCO) over a nanosized Pt-Rh/γ-Al2O3 catalyst prepared by the incipient wetness impregnation method of hexachloroplatinic acid (H2PtCl6) and rhodium (III) nitrate (Rh(NO3)3) with γ-Al2O3 in a tubular fixed-bed flow quartz reactor (TFBR). The characterization of catalysts were thoroughly measured using transmission electron microscopy (TEM), threedimensional excitation-emission fluorescent matrix (EEFM) spectroscopy, UV-Vis absorption, dynamic lightscattering (DLS), zeta potential meter, and cyclic voltammetry (CV). The results demonstrated that at a temperature of 673K and an oxygen content of 4%, approximately 99% of the NH3 was removed by catalytic oxidation over the nanosized Pt-Rh/γ-Al2O3 catalyst. N2 was the main product in NH3-SCO process. Further, it reveals that the oxidation of NH3 was proceeds by the over-oxidation of NH3 into NO, which was conversely reacted with the NH3 to yield N2. Therefore, the application of nanosized Pt-Rh/γ-Al2O3 catalyst can significantly enhance the catalytic activity toward NH3 oxidation. One fluorescent peak for fresh catalyst was different with that of exhausted catalyst. It indicates that EEFM spectroscopy was proven to be an appropriate and effective method to characterize the Pt clusters in intrinsic emission from nanosized Pt-Rh/γ-Al2O3 catalyst. Results obtained from the CV may explain the significant catalytic activity of the catalysts.
Similar content being viewed by others
References
Galloway J N, Townsend A R, Erisman J W, Bekunda M, Cai Z, Freney J R, Martinelli L A, Seitzinger S P, Sutton M A. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science, 2008, 320(5878): 889–892
Cui X, Zhou J, Ye Z, Chen H, Li L, Ruan M, Shi J. Selective catalytic oxidation of ammonia to nitrogen over mesoporous CuO/RuO2 synthesized by co-nanocasting-replication method. Journal of Catalysis, 2010, 270(2): 310–317
Amblard M, Burch R, Southward BWL. A study of the mechanism of selective conversion of ammonia to nitrogen on Ni/γ-Al2O3 under strongly oxidizing conditions. Catalysis Today, 2000, 59(3–4): 365–371
Wang W, Padban N, Ye Z, Andersson A, Bjerle I. Kinetic of ammonia decomposition in hot gas cleaning. Industrial & Engineering Chemistry Research, 1999, 38(11): 4175–4182
Schmidt-SzaŁowski K, Krawczyk K, Petryk J. The properties of cobalt oxide catalyst for ammonia oxidation. Applied Catalysis A: General, 1998, 175(1–2): 147–157
Liang C, Li W, Wei Z, Xin Q, Li C. Catalytic decomposition of ammonia over nitrided MoNx/α-Al2O3 and NiMoNy/α-Al2O3 catalysts. Industrial & Engineering Chemistry Research, 2000, 39(10): 3694–3697
Hung C M. Decomposition kinetics of ammonia in gaseous stream by a nanoscale copper-cerium bimetallic catalyst. Journal of Hazardous Materials, 2008, 150(1): 53–61
Brüggemann T C, Keil F J. Theoretical investigation of the mechanism of the selective catalytic oxidation of ammonia on Hform zeolites. Journal of Physical Chemistry C, 2009, 113(31): 13860–13876
Zhang L, He H. Mechanism of selective catalytic oxidation of ammonia to nitrogen over Ag/Al2O3. Journal of Catalysis, 2009, 268(1): 18–25
Wang Z, Qu Z, Quan X, Wang H. Selective catalytic oxidation of ammonia to nitrogen over ceria-zirconia mixed oxides. Applied Catalysis A: General, 2012, 411–412(1): 131–138
Song S, Jiang S. Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: the promoting effect of the defects of CNTs on the catalytic activity and selectivity. Applied Catalysis B: Environmental, 2012, 117–118(5): 346–350
Hung CM, Lai WL, Lin J L. Removal of gaseous ammonia in Pt-Rh binary catalytic oxidation. Aerosol and Air Quality Research, 2012, 12(4): 583–591
Hung C M. Preparation, properties and cytotoxicity assessment of nanosized Pt-Rh composite catalyst for the decomposition of gaseous ammonia. Advanced Materials Research, 2011, 160–162: 1285–1290
Hung C M. Application of Pt-Rh complex catalyst: feasibility study on the removal of gaseous ammonia. International Journal of Physical Sciences, 2012, 7(14): 2166–2173
Hung C M. The study of catalytic oxidation ammonia reactivity using bimetallic PtRh particles as catalyst: electrocatalytic and electrochemical behavior. Advanced Science Letters, 2012, 8(1): 578–582
Ohno T, Amirbahman A, Bro R. Parallel factor analysis of excitation-emission matrix fluorescence spectra of water soluble soil organic matter as basis for the determination of conditional metal binding parameters. Environmental Science & Technology, 2008, 42(1): 186–192
Henderson R K, Baker A, Murphy K R, Hambly A, Stuetz R M, Khan S J. Fluorescence as a potential monitoring tool for recycled water systems: a review. Water Research, 2009, 43(4): 863–881
Tang Z, Yu G, Liu D, Xu D, Shen Q. Different analysis techniques for fluorescence excitation-emission matrix spectroscopy to assess compost maturity. Chemosphere, 2011, 82(8): 1202–1208
Anderson J A. Infrared study of CO oxidation over Pt-Rh/Al2O3 catalysts. Journal of Catalysis, 1993, 142(1): 153–165
Choi J H, Park K W, Park I S, Nam W H, Sung Y E. Methanol electro-oxidation and direct methanol fuel cell using Pt/Rh and Pt/Ru/Rh alloy catalysts. Electrochimica Acta, 2004, 50(2–3): 787–790
Stoyanovskii V O, Vedyagin A A, Aleshina G I, Volodin A M, Noskov A S. Characterization of Rh/Al2O3 catalysts after calcination at high temperatures under oxidizing conditions by luminescence spectroscopy and catalytic hydrogenolysis. Applied Catalysis B: Environmental, 2009, 90(1–2): 141–146
Hung C M. Fabrication, characterization, and evaluation of the cytotoxicity of platinum-rhodium nanocomposite materials for use in ammonia treatment. Powder Technology, 2011, 209(1–3): 29–34
Hu Z, Allen FM, Wan C Z, Heck RM, Steger J J, Lakis R E, Lyman C E. Performance and structure of Pt-Rh three-way catalysts: mechanism for Pt/Rh synergism. Journal of Catalysis, 1998, 174(1): 13–21
Mulukutla R S, Shido T, Asakuru K, Kogure T, Iwasawa Y. Characterization of rhodium oxide nanoparticles in MCM-41 and their catalytic performances for NO-CO reactions in excess O2. Applied Catalysis A: General, 2002, 228(1–2): 305–314
Curtin T, Regan F O’, Deconinck C, Knűttle N, Hodnett B K. The catalytic oxidation of ammonia: influence of water and sulfur on selectivity to nitrogen over promoted copper oxide/alumina catalysts. Catalysis Today, 2000, 55(1–2): 189–195
Sobczyk D P, de Jong A M, Hensen E J M, van Santen R A. Activation of ammonia dissociation by oxygen on platinum sponge studied with positron emission profiling. Journal of Catalysis, 2003, 219(1): 156–166
Zhang S, Zhao Y. Facile preparation of organic nanoparticles by interfacial cross-linking of reversed micelles and template synthesis of subnanometer Au-Pt nanoparticles. Nano, 2011, 5(4): 2637–2646
Larrivee E M, Elkins K M, Andrews S E, Nelson D J. Fluorescence characterization of the interaction of Al3+ and Pd2+ with Suwannee River fulvic acid in the absence and presence of the herbicide 2,4-dichlorophenoxyacetic acid. Journal of Inorganic Biochemistry, 2003, 97(1): 32–45
Zhang Y, Geddes C D. Metal-enhanced fluorescence from thermally stable rhodium nanodeposits. Journal of Materials Chemistry, 2010, 20(39): 8600–8606
Wu M L, Lai L B. Synthesis of Pt/Ag bimetallic nanoparticles in water-in-oil microemulsions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 244(1–3): 149–157
Xu R. Progress in nanoparticles characterization: sizing and zeta potential measurement. Particuology, 2008, 6(2): 112–115
Du H Y, Wang C H, Hsu H C, Chang S T, Chen U S, Yen S C, Chen L C, Shih H C, Chen K H. Controlled platinum nanoparticles uniformly dispersed on nitrogen-doped carbon nanotubes for methanol oxidation. Diamond and Related Materials, 2008, 17(4–5): 535–541
Prasad K V, Chavdhari R V. Activity and selectivity of supported Rh catalysts for oxidative carbonylation of aniline. Journal of Catalysis, 1994, 145(1): 204–215
Oliveira R T S, Santos MC, Nascente P A P, Bulhões L O S, Pereira E C. Nanogravimetric and voltammetric studies of a Pt-Rh alloy surface and its behavior for methanol oxidation. International Journal of Electrochemical Science, 2008, 3(8): 970–979
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, CM., Lai, WL. & Lin, JL. Investigation of fluorescence characterization and electrochemical behavior on the catalysts of nanosized Pt-Rh/γ-Al2O3 to oxidize gaseous ammonia. Front. Environ. Sci. Eng. 7, 428–434 (2013). https://doi.org/10.1007/s11783-013-0517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-013-0517-0