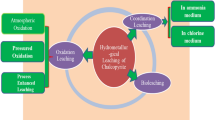
Abstract
Chalcopyrite is one of the most important copper minerals; however, the extracted efficiency of chalcopyrite is still not satisfactory in hydrometallurgy owing to its high lattice energy which leads to its low dissolution kinetics. To overcome the difficulties, many advanced technologies have been developed, including the selection of high effectively bacteria, the inhibition of the passivation film adhered onto the minerals surface, and the maintenance of solution redox potential under an optimum range. Up to date, considerable researches on the first two terms have been summarized, while the overview of the last term has been rarely reported. Based on corresponding works in recent years, key trends and roles of solution redox potential in copper hydrometallurgy, including its definition, effect and maintenance, have been introduced in this review.
摘要
黄铜矿是一种重要的铜资源, 但由于黄铜矿晶格能较高, 溶解动力学较低, 致其在湿法冶金过程中浸出效率不理想. 为了解决这一问题进行了一系列新技术的研究, 包括对高效菌株的选育, 抑制在矿物表面钝化膜的生成, 以及控制溶液氧化还原电位在最佳区间内. 目前, 对前两个技术的研究工作已有大量的总结, 而对于控制氧化还原电位这一过程的工作总结较少. 本文通过研究近年来的相关工作, 介绍了铜湿法冶金过程中溶液氧化还原电位的定义、 作用以及对氧化还原电位的控制等相关内容.
Similar content being viewed by others
References
CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part I: General aspects [J]. Hydrometallurgy, 2008, 93(3, 4): 81–87. DOI: 10.1016/j.hydromet.2008.04.015.
HARMER S L, THOMAS J E, FORNASIERO D, GERSON A R. The evolution of surface layers formed during chalcopyrite leaching [J]. Geochimica et Cosmochimica Acta, 2006, 70(17): 4392–4402. DOI: 0.1016/j.gca.2006.06.1555.
LI Yu-biao, KAWASHIMA N, LI J, CHANDRA A P, GERSON A R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid and Interface Science, 2013, 197: 1–32. DOI: 10.1016/j.cis.2013.03.004.
CHANG Ke-xin, ZHANG Yan-sheng, ZHANG Jia-ming, LI Teng-fei, WANG Jun, QIN Wen-qing. Effect of temperature-induced phase transitions on bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2183–2191. DOI: /10.1016/s1003-6326(19)65124-1.
WANG Jun, HU Ming-hao, ZHAO Hong-bo, TAO Lang, GAN Xiao-wen, QIN Wen-qing, QIU Guan-zhou. Well-controlled column bioleaching of a low-grade copper ore by a novel equipment [J]. Journal of Central South University, 2015, 22(9): 3318–3325. DOI: 10.1007/s11771-015-2872-4.
WATLING H R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options [J]. Hydrometallurgy, 2013, 140: 163–180. DOI: 10.1016/j.hydromet.2013.09.013.
YANG Bao-jun, LUO Wen, WANG Xing-xing, YU Shi-chao, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. The use of biochar for controlling acid mine drainage through the inhibition of chalcopyrite biodissolution [J]. Science of the Total Environment, 2020, 139485. DOI: 10.1016/j.scitotenv.2020.139485.
OLIVEIRA D C, DUARTE H A. Disulphide and metal sulphide formation on the reconstructed (001) surface of chalcopyrite: A DFT study [J]. Applied Surface Science, 2010, 257(4): 1319–1324. DOI: 10.1016/j.apsusc.2010.08.059.
OERTZEN V G, HARMER S L, SKINNER W M. XPS and Ab initio calculation of surface states of sulfide minerals: Pyrite, chalcopyrite and molybdenite [J]. Molecular Simulation, 2006, 32(15): 1207–1212. DOI: 10.1080/08927020601081616.
MIKHLIN Y, TOMASHEVICH Y, TAUSON V, VYALIKH D, MOLODTSOV S, SZARGAN R. A comparative X-ray absorption near-edge structure study of bornite, Cu5FeS4, and chalcopyrite, CuFeS2 [J]. Journal of Electron Spectroscopy and Related Phenomena, 2005, 142(1): 83–88. DOI: 10.1016/j.elspec.2004.09.003.
LLANOS J, BULJAN A, MUJICA C, RAMÍREZ R. Electron transfer in the insertion of alkali metals in chalcopyrite [J]. Materials Research Bulletin, 1995, 30(1): 43–48. DOI: 10.1016/0025-5408(94)00105-7.
VERA M, SCHIPPERS A, SAND W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation-Part A [J]. Applied Microbiology and Biotechnology, 2013, 97(17): 7529–7541. DOI: 10.1007/s00253-013-4954-2.
RAWLINGS D E, JOHNSON D B. Biomining [M]. New York: Springer, 2007.
MISHRA D, KIM D, AHN J G, RHEE Y H. Bioleaching: A microbial process of metal recovery: A review [J]. Metals and Materials International, 2005, 11(3): 249–256. DOI: 10.1007/BF03027450.
BRIERLEY J A, BRIERLEY C L. Present and future commercial applications of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2): 233–239. DOI: 10.1016/S0304-386X(00)00162-6.
ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, ZHENG Xi-hua, TAO Lang, HU Ming-hao, LI Yi-ni, QIN Wen-qing, QIU Guan-zhou. Effects of pyrite and bornite on bioleaching of two different types of chalcopyrite in the presence of Leptospirillum ferriphilum [J]. Bioresoure Technology, 2015, 194: 28–35. DOI: 10.1016/j.biortech.2015.07.003.
JOHNSON D B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials [J]. Current Opinion in Biotechnology, 2014, 30: 24–31. DOI: 10.1016/j.copbio.2014.04.008.
SCHIPPERS A, HEDRICH S, VASTERS J, DROBE M, SAND W, WILLSCHER S. Biomining: Metal recovery from ores with microorganisms [J]. Advances in Biochemical Engineering/Biotechnology, 2014, 141: 1–47. DOI: 10.1007/10_2013_216.
HONG Mao-xin, WANG Xing-xing, WU Ling-bo, FANG Chao-jun, HUANG Xiao-tao, LIAO Rui, ZHAO Hong-bo, QIU Guan-zhou, WANG Jun. Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus [J]. Minerals, 2019, 9(3): 159. DOI: 10.3390/min9030159.
FANG Chao-jun, YU Shi-chao, WANG Xing-xing, ZHAO Hong-bo, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Synchrotron radiation XRD investigation of the fine phase transformation during synthetic chalcocite acidic ferric sulfate leaching [J]. Minerals, 2018, 8(10): 461. DOI: 10.3390/min8100461.
ZHAO Hong-bo, HUANG Xiao-tao, HU Ming-hao, ZHANG Chen-yang, ZHANG Yi-sheng, WANG Jun, QIN Wen-qing, QIU Guan-zhou. Insights into the surface transformation and electrochemical dissolution process of bornite in bioleaching [J]. Minerals, 2018, 8(4): 173. DOI: 10.3390/min8040173.
YANG Cong-ren, QIN Wen-qing, LAI Shao-shi, WANG Jun, ZHANG Yan-sheng, JIAO Fen, REN Liu-yi, ZHUANG Tian, CHANG Zi-yong. Bioleaching of a low grade nickel-copper-cobalt sulfide ore [J]. Hydrometallurgy, 2011, 106(1, 2): 32–37. DOI: 10.1016/j.hydromet.2010.11.013.
ZHEN Shi-jie, YAN Zhong-qiang, ZHANG Yan-sheng, WANG Jun, CAMPBELL Maurice, QIN Wen-qing. Column bioleaching of a low grade nickel-bearing sulfide ore containing high magnesium as olivine, chlorite and antigorite [J]. Hydrometallurgy, 2009, 96(4): 337–341. DOI: 10.1016/j.hydromet.2008.11.007.
QIN Wen-qing, ZHEN Shi-jie, YAN Zhong-qiang, CAMPBELL M, WANG Jun, LIU Kai, ZHANG Yan-sheng. Heap bioleaching of a low-grade nickel-bearing sulfide ore containing high levels of magnesium as olivine, chlorite and antigorite [J]. Hydrometallurgy, 2009, 98(1): 58–65. DOI: 10.1016/j.hydromet.2009.03.017.
ZHEN Shi-jie, QIN Wen-qing, YAN Zhong-qiang, ZHANG Yan-sheng, WANG Jun, REN Liu-yi. Bioleaching of low grade nickel sulfide mineral in column reactor [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1480–1484. DOI: 10.1016/S1003-6326(09)60029-7.
LAN Zhuo-yue, HU Yue-hua, LIU Jian-she, WANG Jun. Solvent extraction of copper and zinc from bioleaching solutions with LIX984 and D2EHPA [J]. Journal of Central South University of Technology, 2005, 12(1): 45–49. DOI: 10.1007/s11771-005-0201-z.
KLAUBER C. Fracture-induced reconstruction of a chalcopyrite (CuFeS2) surface [J]. Surface and Interface Analysis, 2003, 35(5): 415–428. DOI: 10.1002/sia.1539.
HE Huan, XIA Jin-lan, YANG Yi, JIANG Hong-chen, XIAO Chun-qiao, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong, QIU Guan-zhou. Sulfur speciation on the surface of chalcopyrite leached by Acidianus manzaensis [J]. Hydrometallurgy, 2009, 99(1, 2): 45–50. DOI: 10.1016/j.hydromet.2009.06.004.
PANDA S, PARHI P K, NAYAK B D, PRADHAN N, MOHAPATRA U B, SUKLA L B. Two step meso-acidophilic bioleaching of chalcopyrite containing ball mill spillage and removal of the surface passivation layer [J]. Bioresource Technology, 2013, 130: 332–338. DOI: 10.1016/j.biortech.2012.12.071.
ZHAO Hong-bo, WANG Jun, QIN Wen-qing, HU Ming-hao, ZHU Shan, QIU Guan-zhou. Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms [J]. Minerals Engineering, 2015, 71: 159–169. DOI: 10.1016/j.mineng.2014.10.025.
ZHAO Hong-bo, GAN Xiao-wen, WANG Jun, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver-bearing solid waste through a new technique process [J]. Hydrometallurgy, 2017, 171: 374–386. DOI: 10.1016/j.hydromet.2017.06.002.
ZHAO Hong-bo, HUANG Xiao-tao, WANG Jun, LI Yi-ni, LIAO Rui, WANG Xing-xing, QIU Xiao, XIONG Yu-ming, QIN Wen-qing, QIU Guan-zhou. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite [J]. Minerals Engineering, 2017, 109: 153–161. DOI: 10.1016/j.mineng.2017.03.013.
ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, ZHANG Er-xing, QIN Wen-qing, QIU Guan-zhou. Cooperative bioleaching of chalcopyrite and silver-bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis [J]. Minerals Engineering, 2015, 81: 29–39. DOI: 10.1016/j.mineng.2015.07.015.
QIN Wen-qing, ZHANG Yan-sheng, ZHEN Shi-jie, WANG Jun, ZHANG Jian-wen, QIU Guan-zhou. Bioleaching of low-grade copper sulfide ore using a column reactor [J]. Advanced Materials Research, 2009, 71-73: 409–412. DOI: 10.4028/www.scientific.net/AMR.71-73.409.
GERICKE M, GOVENDER Y, PINCHES A. Tank bioleaching of low-grade chalcopyrite concentrates using redox control [J]. Hydrometallurgy, 2010, 104(3, 4): 414–419. DOI: 10.1016/j.hydromet.2010.02.024.
WANG Jun, TAO Lang, ZHAO Hong-bo, HU Ming-hao, ZHENG Xi-hua, PENG Hong, GAN Xiao-wen, XIAO Wei, CAO Pan, QIN Wen-qin, QIU Guan-zhou, WANG Dian-zuo. Cooperative effect of chalcopyrite and bornite interactions during bioleaching by mixed moderately thermophilic culture [J]. Minerals Engineering, 2016, 95: 116–123. DOI: 10.1016/j.mineng.2016.06.006.
OLSON G J, BRIERLEY J A, BRIERLEY C L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 249–257. DOI: 10.1007/s00253-003-1404-6.
ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239–248. DOI: 10.1007/s00253-003-1448-7.
BRIERLEY C L, BRIERLEY J A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries [J]. Applied Microbiology and Biotechnology, 2013, 97(17): 7543–7552. DOI: 10.1007/s00253-013-5095-3.
WANG Xing-xing, LIAO Rui, ZHAO Hong-bo, HONG Mao-xing, HUANG Xiao-tao, PENG Hong, WEN Wen, QIN Wen-qing, QIU Guan-zhou, HUANG Cao-ming, WANG Jun. Synergetic effect of pyrite on strengthening bornite bioleaching by Leptospirillum ferriphilum [J]. Hydrometallurgy, 2018, 176: 9–16. DOI: 10.1016/j.hydromet.2017.12.003.
WANG Jun, ZHAO Hong-bo, QIN Wen-qing, QIU Guan-zhou. Bioleaching of complex polymetallic sulfide ores by mixed culture [J]. Journal of Central South University, 2014, 21(7): 2633–2637. DOI: 10.1007/s11771-014-2223-x.
WANG Jun, ZHAO Hong-bo, ZHUANG Tian, QIN Wen-qing, ZHU Shan, QIU Guan-zhou. Bioleaching of Pb-Zn-Sn chalcopyrite concentrate in tank bioreactor and microbial community succession analysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3758–3762. DOI: 10.1016/s1003-6326(13)62926-x.
CHEN Bo-wei, WU Biao, LIU Xing-yu, WEN Jian-kang. Comparison of microbial diversity during column bioleaching of chalcopyrite at different temperatures [J]. Journal of Basic Microbiology, 2014, 54(6): 491–499. DOI: 10.1002/jobm.201300092.
GU Guo-hua, HU Ke-ting, LI Shuang-ke. Bioleaching and electrochemical properties of chalcopyrite by pure and mixed culture of Leptospirillum ferriphilum and Acidthiobacillus thiooxidans [J]. Journal of Central South University, 2013, 20(1): 178–183. DOI: 10.1007/s11771-013-1474-2.
HUANG Yu-lin, ZHANG Yi-sheng, ZHAO Hong-bo, ZHANG Yan-jun, XIONG Yu-ming, ZHANG Lu-yuan, ZHOU Jun, WANG Jun, QIN Wen-qing, QIU Guan-zhou. Bioleaching of chalcopyrite-bornite and chalcopyrite-pyrite mixed ores in the presence of moderately thermophilic microorganisms [J]. International Journal of Electrochemical Science, 2017, 12(11): 10493–10510. DOI: 10.20964/2017.11.33.
LIANG Yu-ting, ZHU Shan, WANG Jun, AI Chen-bing, QIN Wen-qing. Adsorption and leaching of chalcopyrite by Sulfolobus metallicus YN24 cultured in the distinct energy sources [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 2(6): 549–552. DOI: 10.1007/s12613-015-1106-y.
QIN Wen-qing, LIU Kai, DIAO Meng-xue, WANG Jun, ZHANG Yan-sheng, YANG Cong-ren, JIAO Fen. Oxidation of arsenite (As(III)) by ferric iron in the presence of pyrite and a mixed moderately thermophilic culture [J]. Hydrometallurgy, 2013, 137: 53–59. DOI: 10.1016/j.hydromet.2013.05.011.
RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, GONZALEZ F, MUNOZ J A. Study of bacterial attachment during the bioleaching of pyrite, chalcopyrite, and sphalerite [J]. Geomicrobiology Journal, 2003, 20(2): 131–141. DOI: 10.1080/01490450390193246.
MOUSAVI S M, YAGHMAEI S, VOSSOUGHI M, JAFARI A. Efficiency of copper bioleaching of two mesophilic and thermophilic bacteria isolated from chalcopyrite concentrate of kerman-yazd regions in Iran [J]. Scientia Iranica, 2007, 14(2): 180–184.
MARHUAL N P, PRADHAN N, KAR R N, SUKLA L B, MISHRA B. Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample [J]. Bioresource Technology, 2008, 99(17): 8331–8336. DOI: 10.1016/j.biortech.2008.03.003.
AHMADI A, SCHAFFIE M, MANAFI Z, RANJBAR M. Electrochemical bioleaching of high grade chalcopyrite flotation concentrates in a stirred bioreactor [J]. Hydrometallurgy, 2010, 104(1): 99–105. DOI: 10.1016/j.hydromet.2010.05.001.
LEE J, ACAR S, DOERR D L, BRIERLEY J A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms [J]. Hydrometallurgy, 2011, 105(3, 4): 213–221. DOI: 10.1016/j.hydromet.2010.10.001.
TUPIKINA O V, MINNAAR S H, van HILLE R P, van WYK N, RAUTENBACH G F, DEW D, HARRISON S T L. Determining the effect of acid stress on the persistence and growth of thermophilic microbial species after mesophilic colonisation of low grade ore in a heap leach environment [J]. Minerals Engineering, 2013, 53: 152–159. DOI: 10.1016/j.mineng.2013.07.015.
ABDOLLAHI H, SHAFAEI S Z, NOAPARAST M, MANAFI Z, NIEMELA S I, TUOVINEN O H. Mesophilic and thermophilic bioleaching of copper from a chalcopyrite-containing molybdenite concentrate [J]. International Journal of Mineral Processing, 2014, 128: 25–32. DOI: 10.1016/j.minpro.2014.02.003.
TUPIKINA O V, MINNAAR S H, RAUTENBACH G F, DEW D W, HARRISON S T L. Effect of inoculum size on the rates of whole ore colonisation of mesophilic, moderate thermophilic and thermophilic acidophiles [J]. Hydrometallurgy, 2014, 149: 244–251. DOI: 10.1016/j.hydromet.2013.10.010.
NIE Zhen-yuan, LIU Hong-chang, XIA Jin-lan, YANG Yi, ZHEN Xiang-jun, ZHANG Li-juan, QIU Guan-zhou. Evidence of cell surface iron speciation of acidophilic iron-oxidizing microorganisms in indirect bioleaching process [J]. Biometals, 2016, 29(1): 25–37. DOI: 10.1007/s10534-015-9893-1.
AKCIL A, CIFTCI H, DEVECI H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate [J]. Minerals Engineering, 2007, 20(3): 310–318. DOI: 10.1016/j.mineng.2006.10.016.
ABDOLLAHI H, NOAPARAST M, SHAFAEI S Z, MANAFI Z, MUNOZ J A, TUOVINEN O H. Silver-catalyzed bioleaching of copper, molybdenum from a chalcopyrite-molybdenite concentrate [J]. International Biodeterioration & Biodegradation, 2015, 104: 194–200. DOI: 10.1016/j.ibiod.2015.05.025.
YANG Yi, HARMER S, CHEN Miao. Synchrotron X-ray photoelectron spectroscopic study of the chalcopyrite leached by moderate thermophiles and mesophiles [J]. Minerals Engineering, 2014, 69: 185–195. DOI: 10.1016/j.mineng.2014.08.011.
BOXALL N J, REA S, LI Jian, MORRIS C, KAKSONEN A H. Effect of high sulfate concentrations on chalcopyrite bioleaching and molecular characterisation of the bioleaching microbial community [J]. Hydrometallurgy, 2017, 168: 32–39. DOI: 10.1016/j.hydromet.2016.07.006.
SANDSTRÖM Å, SHCHUKAREV A, PAUL J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential [J]. Minerals Engineering, 2005, 18(5): 505–515. DOI: 10.1016/j.mineng.2004.08.004.
RUBIO A, FRUTOS F J G. Bioleaching capacity of an extremely thermophilic culture for chalcopyritic materials [J]. Minerals Engineering, 2002, 15(9): 689–694. DOI: 10.1016/S0892-6875(02)00124-3.
D'HUGUES P, FOUCHER S, GALLÉ-CAVALLONI P, MORIN D. Continuous bioleaching of chalcopyrite using a novel extremely thermophilic mixed culture [J]. International Journal of Mineral Processing, 2002, 66(1-4): 107–119. DOI: 10.1016/S0301-7516(02)00004-2.
GERICKE M, PINCHES A, ROOYEN J V V. Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture [J]. International Journal of Mineral Processing, 2001, 62(1-4): 243–255. DOI: 10.1016/S0301-7516(00)00056-9.
GÓMEZ E, BALLESTER A, GONZÁLEZ F, BLÁZQUEZ M L. Leaching capacity of a new extremely thermophilic microorganism, Sulfolobus rivotincti [J]. Hydrometallurgy, 1999, 52(3): 349–366. DOI: 10.1016/S0304-386X(99)00027-4.
ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, PENG Juan-hua, CHEN Miao, TAN S N, CHAO Wei-liang, LIU Xue-duan, ZHANG Yan-sheng. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate [J]. Bioresource Technology, 2010, 101(18): 7068–7075. DOI: 10.1016/j.biortech.2010.04.003.
WANG Jun, QIN Wen-qing, ZHANG Yan-sheng, YANG Cong-ren. Bacterial leaching of chalcopyrite and bornite with native bioleaching microorganism [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1468–1472. DOI: 10.1016/S1003-6326(09)60027-3.
ZHANG Yan-sheng, QIN Wen-qing, WANG Jun, ZHEN Shi-jie, YANG Cong-ren, ZHANG Jian-wen, NAI Shao-shi, QIU Guan-zhou. Bioleaching of chalcopyrite by pure and mixed culture [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1491–1496. DOI: 10.1016/S1003-6326(09)60031-5.
HU Ke-ting, GU Guo-hua, LI Shuang-ke, QIU Guan-zhou. Bioleaching of chalcopyrite by Leptospirillum ferriphilum [J]. Journal of Central South University, 2012, 19(6): 1718–1723. DOI: 10.1007/s11771-012-1198-8.
WANG Jun, QIU Guan-zhou, QIN Wen-qing, ZHANG Yan-sheng. Microbial leaching of marmatite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 937–942. DOI: 10.1016/S1003-6326(06)60355-5.
WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides — A review [J]. Hydrometallurgy, 2006, 84(1, 2): 81–108. DOI: 10.1016/j.hydromet.2006.05.001.
YIN Sheng-hua, WANG Lei-ming, KABWE E, CHEN Xun, YAN Rong-fu, AN Kai, ZHANG Lei, WU Ai-xiang. Copper bioleaching in China: Review and prospect [J]. Minerals, 2018, 8(2): 32. DOI: 10.3390/min8020032.
FANG Jing-hua, LIU Yong, HE Wan-li, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Transformation of iron in pure culture process of extremely acidophilic microorganisms [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1150–1155. DOI: 10.1016/s1003-6326(17)60134-1.
BRIERLEY C L, BRIERLEY J A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries [J]. Applied Microbiology and Biotechnology, 2013, 97(17): 7543–7552. DOI: 10.1007/s00253-013-5095-3
DUTRIZAC J E. Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite [J]. Canadian Metallurgical Quarterly, 1989, 28(4): 337–344. DOI: 10.1179/cmq.1989.28.4.337.
ZHAO Hong-bo, ZHANG Yi-sheng, ZHANG Xian, QIAN Lu, SUN Meng-lin, YANG Yu, ZHANG Yan-sheng, WANG Jun, KIM H, QIU Guan-zhou. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview [J]. Minerals Engineering, 2019, 136: 140–154. DOI: 10.1016/j.mineng.2019.03.014.
FU Kai-bin, LIN Hai, MO Xiao-lan, WANG Han, WEN Hong-wei, WEN Zi-long. Comparative study on the passivation layers of copper sulphide minerals during bioleaching [J]. International Journal of Minerals Metallurgy and Materials, 2012, 19(10): 886–892. DOI: 10.1007/s12613-012-0643-x.
YANG Yi, HARMER S L, CHEN Miao. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite [J]. Hydrometallurgy, 2015, 156: 89–98. DOI: 10.1016/j.hydromet.2015.05.011.
KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. International Journal of Mineral Processing, 2008, 86(1-4): 1–17. DOI: 10.1016/j.minpro.2007.09.003.
YANG Bao-jun, ZHAO Chun-xiao, LUO Wen, LIAO Rui, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/j.jhazmat.2020.122290.
YANG Bao-jun, LIN Mo, FANG Jing-hua, ZHANG Rui-yong, LUO Wen, WANG Xing-xing, LIAO Rui, WU Bai-qiang, WANG Jun, GAN Min. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/j.scitotenv.2019.134175.
YANG Yi, LIU Wei-hua, CHEN Miao. XANES and XRD study of the effect of ferrous and ferric ions on chalcopyrite bioleaching at 30 °C and 48 °C [J]. Minerals Engineering, 2015, 70: 99–108. DOI: 10.1016/j.mineng.2014.08.021.
YANG Yi, LIU Wei-hua, CHEN Miao. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles [J]. Minerals Engineering, 2013, 48: 31–35. DOI: 10.1016/j.mineng.2013.01.010.
WANG Jun, GAN Xiao-wen, ZHAO Hong-bo, HU Ming-hao, LI Kai-yun, QIN Wen-qing, QIU Guan-zhou. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis [J]. Minerals Engineering, 2016, 98: 264–278. DOI: 10.1016/j.mineng.2016.09.008.
WU Shi-fa, YANG Cong-ren, QIN Wen-qing, JIAO Fen, WANG Jun, ZHANG Yan-sheng. Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4110–4118. DOI: 10.1016/s1003-6326(15)64062-6.
KHOSHKHOO M, DOPSON M, SHCHUKAREV A, SANDSTROM A. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution [J]. Hydrometallurgy, 2014, 149: 220–227. DOI: 10.1016/j.hydromet.2014.08.012.
LIU Hong-chang, XIA Jin-lan, NIE Zhen-yuan. Relatedness of Cu and Fe speciation to chalcopyrite bioleaching by Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2015, 156: 40–46. DOI: 10.1016/j.hydromet.2015.05.013.
ZHAO Hong-bo, WANG Jun, HU Ming-hao, QIN Wen-qing, ZHANG Yan-sheng, QIU Guan-zhou. Synergistic bioleaching of chalcopyrite and bornite in the presence of Acidithiobacillus ferrooxidans [J]. Bioresource Technology, 2013, 149(4): 71–76. DOI: 10.1016/j.biortech.2013.09.035.
LI Yu-biao, QIAN Gu-jie, LI Jun, GERSON A R. Kinetics and roles of solution and surface species of chalcopyrite dissolution at 650 mV [J]. Geochimica et Cosmochimica Acta, 2015, 161: 188–202. DOI: 10.1016/j.gca.2015.04.012.
ZHAO Hong-bo, WANG Jun, QIN Wen-qing, HU Ming-hao, QIU Guan-zhou. Electrochemical dissolution of chalcopyrite concentrates in stirred reactor in the presence of Acidithiobacillus ferrooxidans [J]. International Journal of Electrochemical Science, 2015, 10(1): 848–858. DOI: 10.1.1.666.6424.
YANG Yi, HARMER S, CHEN Miao. Synchrotron-based XPS and NEXAF study of surface chemical species during electrochemical oxidation of chalcopyrite [J]. Hydrometallurgy, 2015, 156: 89–98. DOI: 10.1016/j.hydromet.2015.05.011.
YU Run-lan, ZHONG Dai-li, MIAO Lei, WU Fa-deng, QIU Guan-zhou, GU Guo-hua. Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1634–1640. DOI: 10.1016/s1003-6326(11)60907-2.
KAPLUN K, LI Jian-chun, KAWASHIMA N, GERSON A R. Cu and Fe chalcopyrite leach activation energies and the effect of added Fe3+ [J]. Geochimica et Cosmochimica Acta, 2011, 75(20): 5865–5878. DOI: 10.1016/j.gca.2011.07.003.
LI Jian-chun, KAWASHIMA N, KAPLUN K, ABSOLON V J, GERSON A R. Chalcopyrite leaching: The rate controlling factors [J]. Geochimica et Cosmochimica Acta, 2010, 74(10): 2881–2893. DOI: 10.1016/j.gca.2010.02.029.
YANG Cong-ren, QIN Wen-qing, ZHAO Hong-bo, WANG Jun, WANG Xing-jie. Mixed potential plays a key role in leaching of chalcopyrite: Experimental and theoretical analysis [J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1733–1744. DOI: 10.1021/acs.iecr.7b02051.
WANG Jun, LIAO Rui, TAO Lang, ZHAO Hong-bo, ZHAI Rui, QIN Wen-qing, QIU Guan-zhou. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching [J]. Hydrometallurgy, 2017, 169: 152–157. DOI: 10.1016/j.hydromet.2017.01.006.
KHOSHKHOO M, DOPSON M, ENGSTRÖM F, SANDSTRÖM Å. New insights into the influence of redox potential on chalcopyrite leaching behaviour [J]. Minerals Engineering, 2017, 100: 9–16. DOI: 10.1016/j.mineng.2016.10.003.
CORDOBA E M, MUÑOZ J A, BLÁZQUEZ M L, GONZÁLEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria [J]. Hydrometallurgy, 2008, 93: 106–115. DOI: 10.1016/j.hydromet.2007.11.005.
KHOSHKHOO M, DOPSON M, SHCHUKAREV A, SANDSTROM A. Electrochemical simulation of redox potential development in bioleaching of a pyritic chalcopyrite concentrate [J]. Hydrometallurgy, 2014, 144: 7–14. DOI: 10.1016/j.hydromet.2013.12.003.
LOTFALIAN M, RANJBAR M, FAZAELIPOOR M H, SCHAFFIE M, MANAFI Z. The effect of redox control on the continuous bioleaching of chalcopyrite concentrate [J]. Minerals Engineering, 2015, 81: 52–57. DOI: 10.1016/j.mineng.2015.07.006.
QIN Wen-qing, YANG Cong-ren, WANG Jun, ZHANG Yan-sheng, JIAO Fen, ZHAO Hong-bo, ZHU Shan. Effect of Fe2+ and Cu2+ ions on the electrochemical behavior of massive chalcopyrite in bioleaching system [J]. Advanced Materials Research, 2013, 825: 472–476. DOI: 10.4028/www.scientific.net/AMR.825.472.
HIROYOSHI N, TSUNEKAWA M, OKAMOTO H, NAKAYAMA R, KUROIWA S. Improved chalcopyrite leaching through optimization of redox potential [J]. Canadian Metallurgical Quarterly, 2008, 47(3): 253–258. DOI: 10.1179/cmq.2008.47.3.253.
HIROYOSHI N, KITAGAWA H, TSUNEKAWA M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2008, 91(1-4): 144–149. DOI: 10.1016/j.hydromet.2007.12.005.
HIROYOSHI N, KUROIWA S, MIKI H, TSUNEKAWA M, HIRAJIMA T. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions [J]. Hydrometallurgy, 2004, 74(1, 2): 103–116. DOI: 10.1016/j.hydromet.2004.01.003.
HIROYOSHI N, KUROIWA S, MIKI H, TSUNEKAWA M, HIRAJIMA T. Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2007, 87(1, 2): 1–10. DOI: 10.1016/j.hydromet.2006.07.006.
HIROYOSHI N, MIKI H, HIRAJIMA T, TSUNEKAWA M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions [J]. Hydrometallurgy, 2001, 60(3): 185–197. DOI: 10.1016/s0304-386x(00)00155-9.
PETERSEN J, DIXON D G. Competitive bioleaching of pyrite and chalcopyrite [J]. Hydrometallurgy, 2006, 83(1-4): 40–49. DOI: 10.1016/j.hydromet.2006.03.036.
THIRD K A, CORD-RUWISCH R, WATLING H R. Control of the redox potential by oxygen limitation improves bacterial leaching of chalcopyrite [J]. Biotechnology and Bioengineering, 2002, 78(4): 433–441. DOI: 10.1002/bit.10184.
ZHAO Hong-bo, WANG Jun, YANG Cong-ren, HU Ming-hao, GAN Xiao-wen, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: An emphasis on solution compositions [J]. Hydrometallurgy, 2015, 151: 141–150. DOI: 10.1016/j.hydromet.2014.11.009.
BEVILAQUA D, LAHTI-TOMMILA H, GARCIA O Jr, PUHAKKA J A, TUOVINEN O H. Bacterial and chemical leaching of chalcopyrite concentrates as affected by the redox potential and ferric/ferrous iron ratio at 22 °C [J]. International Journal of Mineral Processing, 2014, 132: 1–7. DOI: 10.1016/j.minpro.2014.08.008.
QIN Wen-qing, YANG Cong-ren, LAI Shao-shi, WANG Jun, KAI Liu, BO Zhang. Bioleaching of chalcopyrite by moderately thermophilic microorganisms [J]. Bioresource Technology, 2013, 129(2): 200–208. DOI: 10.1016/j.biortech.2012.11.050.
DELAUNE R D, REDDY K R. Encyclopedia of soils in the environment [M]. Elsevier, 2005. DOI: 10.1016/B0-12-348530-4/00212-5.
VANLOON G W, DUFFY S J. Environmental chemistry: A global perspective [M]. Oxford: Oxford University Press, 2010.
HIROYOSHI N, MIKI H, HIRAJIMA T, TSUNEKAWA M. A model for ferrous-promoted chalcopyrite leaching [J]. Hydrometallurgy, 2000, 57(1): 31–38. DOI: 10.1016/s0304-386x(00)00089-x.
ELSHERIEF A E. The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution [J]. Minerals Engineering, 2002, 15(4): 215–223. DOI: 10.1016/s0892-6875(01)00208-4.
GU Guo-hua, HU Ke-ting, ZHANG Xun, XIONG Xian-xue, YANG Hui-sha. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum [J]. Electrochimica Acta, 2013, 103: 50–57. DOI: 10.1016/j.electacta.2013.04.051.
GU Guo-hua, XIONG Xian-xue, HU Ke-ting, LI Shuang-ke, WANG Chong-qing. Stepwise dissolution of chalcopyrite bioleaching by thermophile A.manzaensis and mesophile L.ferriphilum [J]. Journal of Central South University, 2015, 22(10): 3751–3759. DOI: 10.1007/s11771-015-2919-6.
ZHAO Hong-bo, HU Ming-hao, LI Yi-ni, ZHU Shan, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 303–313. DOI: 10.1016/S1003-6326(15)63605-6.
LIU Hong-chang, NIE Zhen-yuan, XIA Jin-lan, ZHU Hong-rui, YANG Yun, ZHAO Chang-hui, ZHENG Lei, ZHAO Yi-dong. Investigation of copper, iron and sulfur speciation during bioleaching of chalcopyrite by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. International Journal of Mineral Processing, 2015, 137: 1–8. DOI: 10.1016/j.minpro.2015.02.008.
ZENG Wei-min, QIU Guan-zhou, CHEN Miao. Investigation of Cu-S intermediate species during electrochemical dissolution and bioleaching of chalcopyrite concentrate [J]. Hydrometallurgy, 2013, 134: 158–165. DOI: 10.1016/j.hydromet.2013.02.009.
WOODS R, YOON R H, YOUNG C A. Eh-pH diagrams for stable and metastable phases in the copper-sulfur-water system [J]. International Journal of Mineral Processing, 1987, 20(1, 2): 109–120. DOI: 10.1016/0301-7516(87)90020-2.
LEE M S, NICOL M J, BASSON P. Cathodic processes in the leaching and electrochemistry of covellite in mixed sulfate-chloride media [J]. Journal of Applied Electrochemistry, 2008, 38(3): 363–369. DOI: 10.1007/s10800-007-9447-5.
HIROYOSHI N, ARAI M, MIKI H, TSUNEKAWA M, HIRAJIMA T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2002, 63(3): 257–267. DOI: 10.1016/s0304-386x(01)00228-6.
ARCE E A, GONZALEZ I. A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution [J]. International Journal of Mineral Processing, 2002, 67(1-4): 17–28. DOI: 10.1016/s0301-7516(02)00003-0.
VILCAEZ J, SUTO K, INOUE C. Bioleaching of chalcopyrite with thermophiles: Temperature-pH-ORP dependence [J]. International Journal of Mineral Processing, 2008, 88(1, 2): 37–44. DOI: 10.1016/j.minpro.2008.06.002.
VILCAEZ J, YAMADA R, INOUE C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures [J]. Hydrometallurgy, 2009, 96(1, 2): 62–71. DOI: 10.1016/j.hydromet.2008.08.003.
LIANG Chang-li, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, ZHAO Xiao-juan, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong. Characterization of the thermo-reduction process of chalcopyrite at 65 °C by cyclic voltammetry and XANES spectroscopy [J]. Hydrometallurgy, 2011, 107(1, 2): 13–21. DOI: 10.1016/j.hydromet.2011.01.011.
ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, CHEN Miao. Electrochemical behaviour of massive chalcopyrite electrodes bioleached by moderately thermophilic microorganisms at 48 °C [J]. Hydrometallurgy, 2011, 105(3, 4): 259–263. DOI: 10.1016/j.hydromet.2010.10.012.
BEVILAQUA D, DIEZ-PEREZ I, FUGIVARA CS, SANZ F, BENEDETTI A V, GARCIA O. Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by electrochemical impedance spectroscopy and atomic force microscopy [J]. Bioelectrochemistry, 2004, 64(1): 79–84. DOI: 10.1016/j.bioelechem.2004.01.006.
ZHAO Hong-bo, WANG Jun, QIN Wen-qing, ZHENG Xi-hua, TAO Lang, GAN Xiao-wen, QIU Guan-zhou. Surface species of chalcopyrite during bioleaching by moderately thermophilic bacteria [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2725–2733. DOI: 10.1016/s1003-6326(15)63897-3.
LIU Qing-you, CHEN Miao, YANG Yi. The effect of chloride ions on the electrochemical dissolution of chalcopyrite in sulfuric acid solutions [J]. Electrochimica Acta, 2017, 253: 257–267. DOI: 10.1016/j.electacta.2017.09.063.
BEVILAQUA D, LAHTI-TOMMILA H, GARCIA O Jr, PUHAKKA J A, TUOVINEN O H. Bacterial and chemical leaching of chalcopyrite concentrates as affected by the redox potential and ferric/ferrous iron ratio at 22 °C [J]. International Journal of Mineral Processing, 2014, 132: 1–7. DOI: 10.1016/j.minpro.2014.08.008.
GU Guo-hua, HU Ke-ting, LI Shuang-ke. Surface characterization of chalcopyrite interacting with Leptospirillum ferriphilum [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1898–1904. DOI: 10.1016/s1003-6326(14)63269-6.
CÓRDOBA E M, MUÑOZ J A, BLÁZQUEZ M L, GONZÁLEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part III: Effect of redox potential on the silver-catalyzed process [J]. Hydrometallurgy, 2008, 93(3, 4): 97–105. DOI: 10.1016/j.hydromet.2007.11.006.
CÓRDOBA E M, MUÑOZ J A, BLÁZQUEZ M L, GONZÁLEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential [J]. Hydrometallurgy, 2008, 93(3, 4): 88–96. DOI: 10.1016/j.hydromet.2008.04.016.
CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria [J]. Hydrometallurgy, 2008, 93(3, 4): 106–115. DOI: 10.1016/j.hydromet.2007.11.005.
ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential [J]. Hydrometallurgy, 2016, 164: 159–165. DOI: 10.1016/j.hydromet.2016.04.013.
ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, QIN Wen-qing, HU Ming-hao, QIU Guan-zhou. Bioleaching of chalcopyrite and bornite by moderately thermophilic bacteria: An emphasis on their interactions [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(8): 777–787. DOI: 10.1007/s12613-015-1134-7.
BARHOUMI N, OLVERAVARGAS H, OTURAN N, HUGUENOT D, GADRI A, AMMAR S, BRILLAS E, OTURAN M A. Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-fenton process with solid catalyst chalcopyrite [J]. Applied Catalysis B-Environmental, 2017, 209: 637–647. DOI: 10.1016/j.apcatb.2017.03.034.
HUANG Xiao-tao, ZHU Tong-he, DUAN Wei-jian, LIANG Sheng, LI Ge, XIAO Wei. Comparative studies on catalytic mechanisms for natural chalcopyrite-induced fenton oxidation: Effect of chalcopyrite type [J]. Journal of Hazardous Materials, 2020, 381: 120998. DOI: 10.1016/j.jhazmat.2019.120998.
WU Biao, WEN Jian-kang, CHEN Bowei, YAO Guo-cheng, WANG Dian-zuo. Control of redox potential by oxygen limitation in selective bioleaching of chalcocite and pyrite [J]. Rare Metals, 2014, 33(5): 622–627. DOI: 10.1007/s12598-014-0364-6.
CHANDRA A P, GERSON A R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective [J]. Surface Science Reports, 2010, 65(9): 293–315. DOI: 10.1016/j.surfrep.2010.08.003.
RUITENBERG R, HANSFORD G S, REUTER M A, BREED A W. The ferric leaching kinetics of arsenopyrite [J]. Hydrometallurgy, 1999, 52(1): 37–53. DOI: 10.1016/S0304-386X(99)00007-9.
MAY N, RALPH D E, HANSFORD G S. Dynamic redox potential measurement for determining the ferric leach kinetics of pyrite [J]. Minerals Engineering, 1997, 10(11): 1279–1290. DOI: 10.1016/S0892-6875(97)00114-3.
NICOL M, MIKI H, BASSON P. The effects of sulphate ions and temperature on the leaching of pyrite. 2. Dissolution rates [J]. Hydrometallurgy, 2013, 133: 182–187. DOI: 10.1016/j.hydromet.2013.01.009.
CHANDRA A P, GERSON A R. Redox potential (Eh) and anion effects of pyrite (FeS2) leaching at pH 1 [J]. Geochimica et Cosmochimica Acta, 2011, 75(22): 6893–6911. DOI: 10.1016/j.gca.2011.09.020.
SUN He-yun, CHEN Miao, ZOU Lai-chang, SHU Rong-bo, RUAN Ren-man. Study of the kinetics of pyrite oxidation under controlled redox potential [J]. Hydrometallurgy, 2015, 155: 13–19. DOI: 10.1016/j.hydromet.2015.04.003.
WEI Zhen-lun, LI Yu-biao, GAO Hui-min, ZHU Yang-ge, QIAN Gu-jie, YAO Jun. New insights into the surface relaxation and oxidation of chalcopyrite exposed to O2 and H2O: A first-principles DFT study [J]. Applied Surface Science, 2019, 492: 89–98. DOI: 10.1016/j.apsusc.2019.06.191.
ZHAO Hong-bo, WANG Jun, TAO Lang, CAO Pan, YANG Cong-ren, QIN Wen-qing, QIU Guan-zhou. Roles of oxidants and reductants in bioleaching system of chalcopyrite at normal atmospheric pressure and 45 °C [J]. International Journal of Mineral Processing, 2017, 162: 81–91. DOI: 10.1016/j.minpro.2017.04.002.
WANG Jun, ZHU Shan, ZHANG Yan-sheng, ZHAO Hong-bo, HU Ming-hao, YANG Cong-ren, QIN Wen-qing, QIU Guan-zhou. Bioleaching of low-grade copper sulfide ores by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Journal of Central South University, 2014, 21(2): 728–734. DOI: 10.1007/s11771-014-1995-3.
YANG Yi, LIU Wei-hua, BHARGAVA S K, ZENG Wei-min, CHEN Miao. A XANE and XRD study of chalcopyrite bioleaching with pyrite [J]. Minerals Engineering, 2016, 89: 157–162. DOI: 10.1016/j.mineng.2016.01.019.
YANG Y, TAN S N, GLENN A M, HARMER S, BHARGAVA S, CHEN M. A direct observation of bacterial coverage and biofilm formation by Acidithiobacillus ferrooxidans on chalcopyrite and pyrite surfaces [J]. Biofouling, 2015, 31(7): 575–586. DOI: 10.1080/08927014.2015.1073720.
WU Biao, WEN Jian-kang, CHEN Bo-wei, YAO Guo-cheng, WANG Dian-zuo. Control of redox potential by oxygen limitation in selective bioleaching of chalcocite and pyrite [J]. Rare Metals, 2014, 33(5): 622–627. DOI: 10.1007/s12598-014-0364-6.
LI Yu-biao, QIAN Gu-jie, BROWN P L, GERSON A R. Chalcopyrite dissolution: Scanning photoelectron microscopy examination of the evolution of sulfur species with and without added iron or pyrite [J]. Geochimica et Cosmochimica Acta, 2017, 212: 33–47. DOI: 10.1016/j.gca.2017.05.016.
OLVERA O G, QUIROZ L, DIXON D G, ASSELIN E. Electrochemical dissolution of fresh and passivated chalcopyrite electrodes. Effect of pyrite on the reduction of Fe3+ ions and transport processes within the passive film [J]. Electrochimica Acta, 2014, 127: 7–19. DOI: 10.1016/j.electacta.2014.01.165.
RUIZ M C, MONTES K S, PADILLA R. Galvanic effect of pyrite on chalcopyrite leaching in sulfate-chloride media [J]. Mineral Processing and Extractive Metallurgy Review, 2014, 36(1): 65–70. DOI: 10.1080/08827508.2013.868349.
HAN Hai-sheng, SUN Wei, HU Yue-hua, CAO Xue-feng, TANG Hong-hu, LIU Run-qing, YUE Tong. Magnetite precipitation for iron removal from nickel-rich solutions in hydrometallurgy process [J]. Hydrometallurgy, 2016, 165: 318–322. DOI: 10.1016/j.hydromet.2016.01.006.
YUE Tong, HAN Hai-sheng, SUN Wei, HU Yue-hua, CHEN Pan, LIU Run-qing. ow-pH mediated goethite precipitation and nickel loss in nickel hydrometallurgy [J]. Hydrometallurgy, 2016, 165: 238–243. DOI: 10.1016/j.hydromet.2016.03.004.
HUANG Xiao-tao, ZHAO Hong-bo, ZHANG Yi-sheng, LIAO Rui, WANG Jun, QIN Wen-qing, QIU Guan-zhou. A strategy to accelerate the bioleaching of chalcopyrite through the goethite process [J]. Minerals & Metallurgical Processing, 2018, 35(4): 171–175. DOI: 10.19150/mmp.8593.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(51774332, U1932129, 51804350, 51934009) supported by the National Natural Science Foundation of China; Project(2018JJ1041) supported by the Natural Science Foundation of Hunan Province, China
Rights and permissions
About this article
Cite this article
Huang, Xt., Liao, R., Yang, Bj. et al. Role and maintenance of redox potential on chalcopyrite biohydrometallurgy: An overview. J. Cent. South Univ. 27, 1351–1366 (2020). https://doi.org/10.1007/s11771-020-4371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-020-4371-5