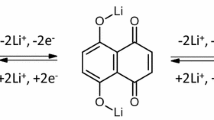
Abstract
Organic electrode materials have high capacity, and environmentally friendly advantages for the next generation lithium-ion batteries (LIBs). However, organic electrode materials face many challenges, such as low reduction potential as cathode materials or high reduction potential as anode materials. Here, the influence of chemical functionalities that are capable of either electron donating or electron withdrawing groups on the reduction potential and charge-discharge performance of anthraquinone (AQ) based system is studied. The cyclic voltammetry results show that the introduction of two —OH groups, two —NO2 groups and one—CH3 group on anthraquinone structure has a little impact on the reduction potential, which is found to be 2.1 V. But when three or four—OH groups are introduced on AQ structure, the reduction potential is increased to about 3.1 V. The charge-discharge tests show that these materials exhibit moderate cycling stability.
摘要
有机电极材料作为下一代锂离子电池材料具有容量高,环境友好型等优势。然而,有机电极材 料面临着许多挑战,如作为正极材料的放电平台低,而作为负极材料的放电平台高。本文研究了具有 供电子和吸电子效应的化学官能团对蒽醌(AQ)体系的还原电位和充放电性能的影响。循环伏安法结果 表明,在蒽醌结构上引入两个—OH 基团、两个—NO2 基团和一个—CH3 基团对蒽醌结构的还原电位 影响较小,还原电位为2.1 V。但在AQ 结构上引入3 ~ 4 个—OH 基团时,还原电位增加到~3.1 V。 充放电测试表明这些材料具有适中的循环稳定性。
Similar content being viewed by others
References
YI Jin, LIU Yang, QIAO Yu, HE Ping, ZHAO Hao-sheng. Boosting the cycle life of Li–O2 batteries at elevated temperature by employing a hybrid polymer–ceramic solid electrolyte [J]. ACS Energy Letters, 2017, 2(6): 1378–1384. DOI: 10.1021/acsenergy lett.7b00292.
QIAO Yu, YI Jin, WU Shu-chao, YANG Si-xie, HE Ping, ZHAO Hao-sheng. Li-CO2 electrochemistry: A new strategy for CO2 fixation and energy storage [J]. Joule, 2017, 1(2): 359–370. DOI: 10.1016/j.joule.2017.07.001.
YI Jin, LIU Xiao-yu, LIANG Peng-cheng, WU Kai, XU Jie, LIU Yu-yu, ZHANG Jiu-jun. Non-noble iron group (Fe, CO, Ni)-based oxide electrocatalysts for aqueous Zinc–air batteries: Recent progress, challenges, and perspectives [J]. Organometallics, 2018, 38(6): 1–14. DOI: 10.1021/acs.organomet.8b00508.
LEI Ping, WANG Yao, ZHANG Fang, WANG Xin, XIANG Xing-de. Carbon-coated Na2.2V1.2Ti0.8(PO4)3 cathode with excellent cycling performance for aqueous Sodium-ion batteries [J]. Chem Electro Chem, 2018, 5(17): 2482–2487. DOI: 10.1002/ celc.201800379.
LI Wan-fang, ZHANG Fang, XIANG Xing-de, ZHANG Xiu-cheng. Electrochemical properties and redox mechanism of Na2Ni0.4CO0.6[Fe(CN)6] nanocrystallites as high-capacity cathode for aqueous sodium-ion batteries [J]. The Journal of Physical Chemistry C, 2017, 121(50): 27805–27812. DOI: 10.1021/acs.jpcc.7b07920.
LIU Yang, YI Jin, QIAO Yu, WANG Di, HE Ping, LI Qi, WU Shi-chao, ZHOU Hao-shen. Solar-driven efficient Li2O2 oxidation in solid-state Li-ion O2 batteries [J]. Energy Storage Materials, 2018, 11: 170–175. DOI: 10.1016/j.ensm.2017.10.002.
WU Shi-chao, YI Jin, ZHU Kai, BAI Song-yan, LIU Yang, QIAO Yu, ISHIDA M, ZHOU Hao-shen. A superhydrophobic quasi-Solid electrolyte for Li-O2 battery with improved safety and cycle life in humid atmosphere[J]. Advanced Energy Materials, 2017, 7(4): 1601759. DOI: 10.1002/aenm.201601759.
LEE J, URBAN A, LI Xin, SU Dong, HAUTIER G, CEDER G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries [J]. Science, 2014, 343(6170): 519–522. DOI: 10.1126/science.1246432.
LI Lei, RAJI A O, TOUR J M. Graphene-wrapped MnO2–graphene nanoribbons as anode materials for high-performance lithium ion batteries [J]. Advanced Materials, 2013, 25(43): 6298–6302. DOI: 10.1002/adma.201302915.
HUANG Jun-da, WEI Zeng-xi, LIAO Jia-qin, NI Wei, WANG Cai-yun, MA Jian-min. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MOS2 [J]. Journal of Energy Chemistry, 2018, 33(7): 1–25. DOI: 10.1016/j.jechem.2018.09.001.
LEI Kai-xiang, WANG Chen-chen, LIU Luo-jia, LUO Yu-wen, MU Chao-nan, LI Fun-jun, CHEN Jun. A porous network of bismuth used as the anode material for high-energy-density Potassium-ion batteries [J]. Angewandte Chemie International Edition, 2018, 130(17): 4777–4781. DOI: 10.1002/ange.201801389.
GUO Zhao-wei, MA Yuan-yuan, DONG Xiao-li, HUANG Jian-hang, WANG Yong-gang, XIA Yong-yao. An environmentally friendly and flexible aqueous Zinc battery using an organic cathode [J]. Angewandte Chemie International Edition, 2018, 57(36): 11737–11741. DOI: 10.1002/ange.201807121.
YI Jin, LIANG Peng-cheng, LIU Xiao-yu, WANG Yong-gang, XIA Yong-yao, ZHANG Jiu-jun. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc–air batteries [J]. Energy & Environmental Science, 2018, 11(11): 3075–3095. DOI: 10.1039/C8EE01991F.
LUO Zhi-qiang, LIU Luo-jia, NING Jia-xin, LEI Kai-xiang, LU Yong, LI Fun-jun, CHEN Jun. A microporous covalent organic framework with abundant accessible carbonyls for lithium-ion batteries [J]. Angewandte Chemie International Edition, 2018, 57(30): 1–6. DOI: 10.1002/anie.201805540.
BACHMAN J E, CURTISS L A, ASSARY R S. Investigation of the redox chemistry of anthraquinone derivatives using density functional theory [J]. Journal of Physical Chemistry A, 2014, 118(38): 8852–8860. DOI: 10.1021/jp5060777.
HAUPLER B, WILD A, SCHUBERT U S. Carbonyls: powerful organic materials for secondary batteries [J]. Advanced Energy Materials, 2015, 5(11): 1402034. DOI: 10.1002/aenm.201402034.
WANG Heng-guo, SHUANG Yuan, MA De-long, HUANG Xiao-lei. Tailored aromatic carbonyl derivative polyimides for high-power and long-cycle sodium-organic batteries [J]. Advanced Energy Materials, 2014, 4: 1301651. DOI: 10.1002/aenm.201301651.
LIANG Yan-liang, ZHANG Peng, YANG Si-qi, TAO Zhan-liang. Fused heteroaromatic organic compounds for high-power electrodes of rechargeable lithium batteries [J]. Advanced Energy Materials, 2013, 3(5): 600–605. DOI: 10.1002/aenm.201200947.
XING Li-dan, ZHENG Xiong-wen, SCHROEDER M, ALVARADO J, CRESCE A W, XU Kang, LI Qiao-shu, LI Wei-shan. Deciphering the ethylene carbonate–propylene carbonate mystery in Li-ion batteries [J]. Accounts of Chemical Research, 2018, 51(2): 282–289. DOI: 10.1021/acs.accounts.7b00474.
LEE Y G, RYU K S, CHANG S H. Chemically synthesized high molecular weight poly(2,2’-dithiodianiline) (PDTDA) as a cathode material for lithium rechargeable batteries [J]. Journal of Power Sources, 2003, 119: 321–325. DOI: 10.1016/S0378-7753(03)00146-0.
LI Jin-xia, ZHAN Hui, ZHOU Lei, DENG Shi-ren, LI Zhao-ying, ZHOU Yun-hong. Aniline-based polyorganodisulfide redox system of high energy for secondary lithium batteries [J]. Electrochemistry Communications, 2004, 6(6): 515–519. DOI: 10.1016/j.elecom.2004.03.010.
NISHIDE H, IWASA S, PU Y J, SUGA T, NAKAHARA K, SATOH M. Organic radical battery: nitroxide polymers as a cathode-active material [J]. Electrochimica Acta, 2004, 50(2): 827–831. DOI: 10.1016/j.electacta.2004.02.052.
YAO M, ANDO H, KIYOBAYASHI T. Polycyclic quinone fused by a sulfur-containing ring as an organic positiveelectrode material for use in rechargeable lithium batteries [J]. Energy Procedia, 2016, 89: 222–230. DOI: 10.1016/j.egypro.2016.05.029.
XUE Long-jian, LI Jin-xia, HU Su-qing, ZHANG Ming-xia, ZHOU Yun-hong, ZHAN Cai-mao. Anthracene based organodisulfide positive active materials for lithium secondary battery [J]. Electrochemistry Communications, 2003, 5(10): 903–906. DOI: 10.1016/j.elecom.2003.08.018.
WAN Wang, LEE Hung-sui, YU Xi-qian, WANG Chao. Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group [J]. RSC Advances, 2014, 4(38): 19878–19882. DOI: 10.1039/c4ra01166j.
CHEN Hai-yan, POIZOT P, DOLHEM F, BASIR N I, MENTRE O, TARASCON J M. Electrochemical reactivity of lithium chloranilate vs Li and crystal structures of the hydrated phases [J]. Electrochemical and Solid-State Letters, 2009, 12(5): A102–A106. DOI: 10.1149/1.3082038.
YOKOJI T, MATSUBARA H, SATOHB M. Rechargeable organic lithium-ion batteries using electron-deficient benzoquinones as positive-electrode materials with high discharge voltages [J]. Journal of Materials Chemistry A, 2014, 2: 19347–19354. DOI: 10.1039/C4TA02812K.
BANDA H, DAMIEN D, NAGRAJAN K, RAJ A, HARUARAN M, SHAIJUMON M. Sodium-ion batteries: Twisted perylene diimides with tunable redox properties for organic sodium-ion batteries [J]. Advanced Energy Materials, 2017, 7(20): 1701316. DOI: 10.1002/aenm.201770112.
ZENG Rong-hua, XING Li-dan, QIU Yong-cai, WANG Ya-ting, HUANG Wan-na, LI Wei-shan, YANG Shi-he. Polycarbonyl(quinonyl) organic compounds as cathode materials for sustainable lithium ion batteries [J]. Electrochimica Acta, 2014, 146: 447–454. DOI: 10.1016/j.electacta.2014.09.082.
HAN Xiao-yan, CHANG Cai-xian, YUAN Liang-jie, SUN Tao-lei. Aromatic carbonyl derivative polymers as high performance Li-ion storage materials [J]. Advanced Materials, 2010, 19(12): 1616–1621. DOI: 10.1002/adma. 200790044.
XIONG Jiang-feng, CHANG Cai-xian, LI Ming, WU Si-min. A novel coordination polymer as positive electrode material for lithium ion battery [J]. Crystal Growth & Design, 2008, 8(1): 280–282. DOI: 10.1021/cg070386q.
ZHOU Qing, ZHU Zhi-qiang, CHEN Jun. Molecular engineering with organic carbonyl electrode materials for advanced stationary and redox flow rechargeable batteries [J]. Advanced Materials, 2017, 29(48). DOI: 10.1002/adma.201607007.
WU Yi-wen, ZENG Rong-hua, NAN Jun-min, SHU Dong. Quinone electrode materials for rechargeable lithium/sodium ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1700278. DOI: 10.1002/aenm.201700278.
PIRNAT K, DOMINKO R, CERE- KOROSEC R, MALI G, GENORIO B, GABERSCSK M. Electrochemically stabilised quinone based electrode composites for Li-ion batteries [J]. Journal of Power Sources, 2012, 199(1): 308–314. DOI: 10.1016/j.jpowsour.2011.10.068.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Foundation item: Project(21875076) supported by the National Natural Science Foundation of China; Projects(2018A050506077, 2017A050506048) supported by the Scientific and Technological Plan of Guangdong Province, China; Project(201910574037) supported by the Undergraduates' Innovating Experimentation Project of China
Rights and permissions
About this article
Cite this article
Qian, Sh., Pan, Jx., Zhu, Zs. et al. Structural modulation of anthraquinone with different functional groups and its effect on electrochemical properties for lithium-ion batteries. J. Cent. South Univ. 26, 1449–1457 (2019). https://doi.org/10.1007/s11771-019-4101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4101-z