Abstract
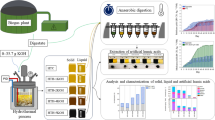
Waste cellulosic biomass obtains various applications due to low-cost and eco-benign characteristics. A general strategy is proposed for waste cellulosic biomass to be modified with dialdehyde functional groups as intermediates through periodate partial oxidation. Finally, aminothiourea-modified waste cellulosic biomass can be prepared through Schiff reaction. Waste corn stalk, cotton and paper as typical precursors, were used to prepare cellulosic biomass, abbreviated as AT-S, AT-C and AT-P, respectively, and their adsorption behaviors of Au(III) from the hydrochloric acid medium were investigated. The pseudo-second kinetics equation as well as the Langmuir isotherm equation can be used to depict the adsorption process, and the maximum adsorption capacities of Au(III) are 21.4, 19.0 and 3.28 mol/kg for AT-S, AT-C and AT-P at 298 K, respectively. The adsorption capacities of Au(III) on aminothiourea modified corn stalk (AT-S) is almost 357 times greater than that of raw corn stalk. To the best of our knowledge, AT-S has the highest adsorption capacity towards Au(III). AT-S also displays a superior separation selectivity towards Au(III) in the presence of Cu(II), Ni(II), Co(II), Pt(VI), Pd(II) and Rh(III). Furthermore, the characterization analysis of XRD, TG, SEM, TEM and FTIR confirms that AuCl4– has been reduced to elemental Au nanoparticles and deposit onto the surface of the biomass. It shows a prospect for waste corn stalk to be used to adsorb Au(III) from liquid phase and the possible fabrication of gold nanoparticles by a general adsorption process without any reductant.
摘要
纤维素基废弃生物质具有价格低廉和环境友好等优点,因而得到了广泛应用。本文提出了一种 纤维素基废弃生物质功能化的通用制备方法,即先通过NaIO4 选择性氧化将废弃生物质转化为含双醛 基的平台中间体,然后利用醛基特定的Schiff base 反应引入对金离子有优良配位能力的配基。以典型 的废弃纤维素基生物质,如玉米秸秆 (AT-S)、棉花 (AT-C) 和纸 (AT-P) 为原料制备了氨基硫脲修饰 的吸附剂。AT-S、AT-C 和AT-P 在298 K 下对Au(III) 的饱和吸附容量分别为21.4、19.0 和3.28 mol/kg, Au(III)吸附量与吸附剂中硫脲官能团的含量成正相关性。Au(III)的吸附符合准二级动力学吸附模型和 Langmuir 等温吸附模型。AT-S 对Au(III)的吸附量是玉米秸秆的357 倍,是目前文献中报道的有关Au(III) 吸附容量最高的吸附剂。AT-S 对Au(III)与其他金属如Cu(II)、Ni(II)、Co(II)、Pt(VI)、Pd(II)和Rh(III) 有着优良的分离选择性。采用XRD、TG、SEM、TEM 和FTIR 进行分析表征,结果表明吸附的AuCl4– 被还原为Au0 纳米颗粒并沉积在AT-S 吸附剂表面。
Similar content being viewed by others
References
DAS N. Recovery of precious metals through biosorption — A review [J]. Hydrometallurgy, 2010, 103(1–4): 180–189.
LI X B, YE J J, LIU Z H, QIU Y Q, LI L J, MAO S, WANG X C, ZHANG Q. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution [J]. Journal of Central South University, 2018, 25(1): 9–20.
TIAN Q H, WANG X Y, MAO F F, GUO X Y. Absorption performance of DMSA modified Fe3O4@SiO2Core/Shell magnetic nanocomposite for Pb2+ Removal [J]. Journal of Central South University, 2018; 25(4): 709–718.
LI J H, MIAO X X, CHEN X Y, LU L, YANG Y, FU Y Q, XIONG C H. Application and characterization of grafted polytetrafluoroethylene fiber for enhanced adsorption of Cu(II) in aqueous solutions [J]. Journal of Central South University, 2016, 23(10): 2513–2519.
PANGENI B, PAUDYAL H, ABE M, INOUE K, KAWAKITA H, OHTO K, ADHIKARI B B, ALAM S. Selective recovery of gold using some cross linked polysaccharide gels [J]. Green Chem, 2012, 14(7): 1917–1927.
GURUNG M, ADHIKARI B B, GAO X, ALAM S, INOUE K. Sustainability in the metallurgical industry: Chemically modified cellulose for selective biosorption of gold from mixtures of base metals in chloride media [J]. Ind Eng Chem Res, 2014, 53(20): 8565–8576.
PARAJULI D, ADHIKARI C R, KAWAKITA H, KAJIYAMA K, OHTO K, INOUE K. Reduction and accumulation of Au(III) by grape waste: A kinetic approach [J]. React Funct Polym, 2008, 68(8): 1194–1199.
XIONG Y, ADHIKARI C R, KAWAKITA H, OHTO K, INOUE K, HARADA H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine [J]. Bioresour Technol, 2009, 100(18): 4083–4089.
FAN R, FENG X, GUAN X, ZHANG Q, LUO Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from E-Wastes [J]. Bioresour Technol, 2014, 163(7): 161–171.
PING Y, XU M, QU R, HOU C, LIU X, JIANG Z, QIANG X. Uptake of Gold (III) from waste gold solution onto biomass-based adsorbents organophosphonic acid functionalized spent buckwheat hulls [J]. Bioresour Technol, 2013, 128(1): 36–43.
PANGENI B, PAUDYAL H, INOUE K, KAWAKITA H, OHTO K, ALAM S. An Assessment of gold recovery processes using cross-linked paper gel [J]. Journal of Chemical and Engineering Data, 2012, 57(3): 381–391.
PANGENI B, PAUDYAL H, INOUE K, KAWAKITA H, OHTO K, ALAM S. Selective recovery of Gold(III) using cotton cellulose treated with concentrated sulfuric acid [J]. Cellulose, 2012, 19(2): 381–391.
ZUO G, ORECCHIO S, MUHAMMED M. Facilitated transport of gold through a membrane via complexation to thiourea-based reagents [J]. Sep Sci Technol, 1996, 31(11): 1597–1613.
ZUO G, MUHAMMED M. Thiourea-based coordinating polymers: Synthesis and binding to noble metals [J]. React Polym, 1995, 24(3): 165–181.
YIRIKOGLU H, GULFEN M. Separation and recovery of Silver(I) ions from base metal ions by melamine-formaldehyde-thiourea (MFT) chelating resin [J]. Sep Sci Technol, 2008, 43(2): 376–388.
JACKSON E L, HUDSON C S. Application of the cleavage type of oxidation by periodic acid to starch and cellulose [J]. J Am Chem Soc, 1937, 59(10): 2049–2050.
ZHENG L, DANG Z, YI X, ZHANG H. Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk [J]. J Hazard Mater, 2010, 176(1–3): 650–656.
CORDES E H, JENCKS W P. On the mechanism of schiff base formation and hydrolysis [J]. J Am Chem Soc, 1962, 84(5): 832–837.
LANGMUIR I. The constitution and fundamental properties of solids and liquids. Part I. Solids [J]. J Am Chem Soc, 1916, 38(11): 2221–2295.
FREUNDLICH H M F. Ueber die adsorption in lesungen [J]. Z Phys Chem, 1906, 57A: 385–470. (in Germany)
HE Z W, HE L H, YANG J, LÜ Q F. Removal and recovery of Au(III) from aqueous solution using a low-cost lignin-based biosorbent [J]. Ind Eng Chem Res, 2013, 52(11): 4103–4108.
GURUNG M, ADHIKARI B B, KAWAKITA H, OHTO K, INOUE K, ALAM S. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract [J]. Chem Eng J, 2011, 174(2, 3): 556–563.
PARAJULI D, KHUNATHAI K, ADHIKARI C R, INOUE K, OHTO K, KAWAKITA H, FUNAOKA M, HIROTA K. Total recovery of gold, palladium, and platinum using lignophenol derivative [J]. Miner Eng, 2009, 22(13): 1173–1178.
DONIA A M, ATIA A A, ELWAKEEL K Z. Recovery of Gold(III) and silver(I) on a chemically modified chitosan with magnetic properties [J]. Hydrometallurgy, 2007, 87(3, 4): 197–206.
LAGERGREN S. Zur theorie der sogenannten adsorption gelöster stoffe [J]. K Sven Vetenskapsakad Handl, 1898, 24(4): 1–39. (in Swedish)
HO Y S. Adsorption of Heavy Metals from Waste Streams by Peat [D]. Birmingham, UK: University of Birmingham, 1995.
YU J, CHENG R. Precious metal extraction chemistry [M]. Beijing, China: Beijing Chemical Industry Press, 2010. (in Chinese)
SPEIGHT J G. Lange’s handbook of chemistry [M]. 16th Edition, New York: McGraw-Hil, 1998.
MATA Y N, TORRES E, BLÁZQUEZ M L, BALLESTER A, GONZÁLEZ F, MUÑOZ J A. Gold(III) biosorption and bioreduction with the brown alga fucus vesiculosus [J]. J Hazard Mater, 2009, 166(2, 3): 612–618.
OGATA T, NAKANO Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin [J]. Water Res, 2005, 39(18): 4281–4286.
WANG F, ZHAO J, ZHU M, YU J, HU Y S, LIU H. Selective adsorption-deposition of gold nanoparticles onto monodispersed hydrothermal carbon spherules: A reduction-deposition coupled mechanism [J]. J Mater Chem A, 2015, 3(4): 1666–1674.
WANG S. Synthesis of Polymeric Ester Thiourea Resin and Its Adsorption and Separation Properties for Noble Metal Ions [D]. Changsha, China: Central South University, 2008. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(51504073, 51404081, 51672275) supported by the National Natural Science Foundation of China; Project(2012CBA01202) supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology, China; Project (QianJiaoKeHe KY[2015]433) supported by the Research Program of the Education Department of Guizhou Province, China; Project(XJG20141104) supported by the Research Program of Talented Scholars of Guizhou Institute of Technology, China
Rights and permissions
About this article
Cite this article
Wang, Fc., Zhao, Jm., Wang, Wk. et al. Superior Au-adsorption performance of aminothiourea-modified waste cellulosic biomass. J. Cent. South Univ. 25, 2992–3003 (2018). https://doi.org/10.1007/s11771-018-3969-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-018-3969-3