Abstract
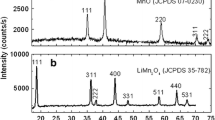
A simple hydrothermal process followed by heat treatment was applied to the preparation of spinel Li1.05Mn1.95O4. In this process, electrolytic manganese dioxide (EMD) and LiOH·H2O were used as starting materials. The physiochemical properties of the synthesized samples were investigated by thermogravimetry-differential scanning calorimetry (TG-DSC), X-ray diffractometry (XRD), and scanning electronic microscopy (SEM). The results show that the hydrothermally synthesized precursor is an essential amorphous. The precursor can be easily transferred to spinel powders with a homogeneous structure and a regularly-shaped morphology by heat treatment. Li1.05Mn1.95O4 powder obtained by heat treating the precursor at 430 °C for 12 h and then calcining at 800 °C for 12 h shows an excellent cycling performance with an initial charge capacity of 118.2 mA·h·g−1 obtained at 0.5C rate and 93.8% of its original value retained after 100 cycles.
Similar content being viewed by others
References
WINTER M, BESENHARD J O, SPAHR M E, NOV K P. Insertion electrode materials for rechargeable lithium batteries [J]. Advanced Materials, 1998, 10(10): 725–763
DAHN J R, von SACKEN U, MICHAL C A. Structure and electrochemistry of Li1±y NiO2 and a new Li2NiO2 phase with the Ni(OH)2 structure [J]. Solid State Ionics, 1990, 44(1/2): 87–97.
DING Y L, XIE J, CAO G S, ZHU T J, YU H M, ZHAO X B. Enhanced elevated-temperature performance of Al-doped single-crystalline LiMn2O4 nanotubes as cathodes for lithium ion batteries [J]. The Journal of Physical Chemistry C, 2011, 115(19): 9821–9825
HASSOUN J, LEE K-S, SUN Y-K, SCROSATI B. An advanced lithium ion battery based on high performance electrode materials [J]. Journal of the American Chemical Society, 2011, 133(9): 3139–3143
NISHIDA Y, NAKANE K, SATOH T. Synthesis and properties of gallium-doped LiNiO2 as the cathode material for lithium secondary batteries [J]. Journal of Power Sources, 1997, 68(2): 561–564
ARAI H, OKADA S, OHTSUKA H, ICHIMURA M, YAMAKI J. Characterization and cathode performance of Li1-x Ni1+x O2 prepared with the excess lithium method [J]. Solid State Ionics, 1995, 80(3/4): 261–269.
OHZUKU T, UEDA A, KOUGUCHI M. Synthesis and characterization of LiAl1/4Ni3/4O2 (R[overline 3]m) for lithium-ion (shuttlecock) batteries [J]. Journal of the Electrochemical Society, 1995, 142(12): 4033–4039.
ARAI H, OKADA S, SAKURAI Y, YAMAKI J I. Thermal behavior of Li1−y NiO2 and the decomposition mechanism [J]. Solid State Ionics, 1998, 109(3/4): 295–302.
BROUSSELY M, PERTON F, BIENSAN P, BODET J M, LABAT J, LECERF A, DELMAS C, ROUGIER A, PERES J P. LixNiO2, a promising cathode for rechargeable lithium batteries [J]. Journal of Power Sources, 1995, 54(1): 109–114.
LE GOFF P, BAFFIER N, BACH S, PEREIRA-RAMOS J P. Synthesis, ion exchange and electrochemical properties of lamellar phyllomanganates of the birnessite group [J]. Materials Research Bulletin, 1996, 31(1): 63–75.
THACKERAY M M. Manganese oxides for lithium batteries [J]. Progress in Solid State Chemistry, 1997, 25(1/2): 1–71.
KANG K, MENG Y S, br GER J, GREY C P, CEDER G. Electrodes with high power and high capacity for rechargeable lithium batteries [J]. Science, 2006, 311(5763): 977–980.
KIM D K, MURALIDHARAN P, LEE H-W, RUFFO R, YANG Y, CHAN C K. Spinel LiMn2O4 nanorods as lithium ion battery cathodes [J]. Nano Letters, 2008, 8(11): 3948–3952.
IQBAL M J, ZAHOOR S. Synthesis and characterization of nanosized lithium manganate and its derivatives [J]. Journal of Power Sources, 2007, 165(1): 393–397.
SINHA N N, RAGUPATHY P, VASAN H N, MUNICHANDRAIAH N. Electrochemical characterization of submicron size particles of LiMn2O4 in aqueous electrolytes [J]. International Journal of Electrochemical Science, 2008, 3: 691–710.
LUO J Y, XIONG H M, XIA Y Y. LiMn2O4 nanorods, nanothorn microspheres, and hollow nanospheres as enhanced cathode materials of lithium ion battery [J]. The Journal of Physical Chemistry C, 2008, 112(31): 12051–12057.
CUI T H N, HAN Y, KANG X. Preparation and electrochemical properties of LiMn2O4 by a rheological-phase-assisted microwave synthesis method [J]. Inorg Mater, 2008, 44: 542–548.
BYRAPPA K, ADSCHIRI T. Hydrothermal technology for nanotechnology [J]. Progress in Crystal Growth and Characterization of Materials, 2007, 53(2): 117–166.
LI Y J, KONG L, XI X M, LI W J, LI G L, LI J Q. Hydrothermal preparation and characterization of LiMn2O4 for Li-ion battery application [C]// Proceedings of the 51st Annual Conference of Metallurgists of CIM (COM 2012). Niagara Falls, Ontario, Canada, 2012: 399–407.
SCHILLING O, DAHN J R. Fits of the γ-MnO2 structure model to disordered manganese dioxides [J]. Journal of Applied Crystallography, 1998, 31(3): 396–406.
YAMADA A, MIURA K, HINOKUMA K, TANAKA M. Synthesis and structural aspects of LiMn2O4±δ as a cathode for rechargeable lithium batteries [J]. ChemInform, 1995, 26(47): 2149–2156.
JIANG C H, DOU S X, LIU H K, ICHIHARA M. Synthesis of spinel LiMn2O4 nanoparticles through one-step hydrothermal reaction [J]. Journal of Power Sources, 2007, 172(1): 410–415.
THACKERAY M M, JOHNSON P J, de PICCIOTTO L A, BRUCE P G, GOODENOUGH J B. Electrochemical extraction of lithium from LiMn2O4 [J]. Materials Research Bulletin, 1984, 19(2): 179–187.
YI T, DAI C, GAO K, HU X. Effects of synthetic parameters on structure and electrochemical performance of spinel lithium manganese oxide by citric acid-assisted sol-gel method [J]. Journal of Alloys and Compounds, 2006, 425(1/2): 343–347.
HON Y M, FUNG K Z, LIN S P, HON M H. Effects of metal ion sources on synthesis and electrochemical performance of spinel LiMn2O4 using tartaric acid gel process [J]. Journal of solid state chemistry, 2002, 163(1): 231–238.
HUNG F Y, LUI T S, LIAO H C. A study of nano-sized surface coating on LiMn2O4 materials [J]. Applied Surface Science, 2007, 253(18): 7443–7448.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(50174058) supported by the National Natural Science Foundation of China; Project(2011A025) supported by the Glorious Laurel Scholar Program of Guangxi Zhuang Autonomous Region, China
Rights and permissions
About this article
Cite this article
Kong, L., Li, Yj., Zhang, P. et al. Performances of lithium manganese oxide prepared by hydrothermal process. J. Cent. South Univ. 21, 1279–1284 (2014). https://doi.org/10.1007/s11771-014-2063-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-014-2063-8