Abstract
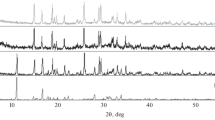
The dissolution mechanism of hemimorphite in NH3-(NH4)2SO4-H2O system at 298.15 K was investigated by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) analysis. The results show that hemimorphite is soluble in NH3-(NH4)2SO4-H2O system and its residue exists in the form of an amorphous SiO2 layer on the hemimorphite surface. The XPS data also indicate that the Si 2p3/2 and O 1s spectra of the hemimorphite are broadened and shift to higher binding energies and their binding energies are closer to silica with an increase of total ammonia and time. Solubility of hemimorphite in NH3-(NH4)2SO4-H2O system was measured by means of isothermal solution method at 298.15 K based on the study of the dissolution mechanism of hemimorphite. The results show that the solubility of zinc in solution increases firstly and then decreases with the increase of c T(NH3) (total ammonia concentration) at different NH3/NH4 + ratios. The solubility of silicon in solution decreases from 0.0334 mol/kg in c T(NH3)=4.1245 mol/kg NH3-(NH4)2SO4-H2O solution to 0.0046 mol/kg in c T(NH3)=7.6035 mol/kg NH3-(NH4)2SO4-H2O solution.
Similar content being viewed by others
References
TERRY B, MONHEMIUS A J. Acid dissolution of willemite ((Zn, Mn)2SiO4) and hemimorphite Zn4Si2O7(OH)2.H2O [J]. Metallurgical and Materials Transactions B [J]. 1983, 14(3): 335–346.
FRENAY J. Leaching of oxidized zinc ores in various media [J]. Hydrometallurgy, 1985, 15(2): 243–253.
ABDEL-AAL E A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore [J]. Hydrometallurgy, 2000, 55: 247–254.
KUMAR R, BISWAS A K. Zinc recovery from Zawar ancient siliceous slag [J]. Hydrometallurgy, 1986, 15(3): 267–280.
ESPIARI S, RASHCHI F, SADMEZHAAD S K. Hydrometallurgical treatment of tailings with high zinc content [J]. Hydrometallurgy, 2006, 82: 54–62.
LI Cun-xiong, XU Sheng, DENG Zhi-gan, LI Xing-bin, LI Ming-tin, WEI Chang. Pressure leaching of zinc silicate ore in sulfuric acid medium [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 918–923.
CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUO Guang-sheng, CHEN Xing-yu. Alkeline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97: 228–232.
ZHAO Zhong-wei, LONG Shuang, CHEN Ai-liang, HUO Guang-sheng, LI Hong-gui, JIA Xi-jun, CHEN Xing-yu. Mechanochemical leaching of refractory zinc silicate (hemimorphite) in alkaline solution [J]. Hydrometallurgy, 2009, 99: 255–258.
DING Zhi-ying, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution [J]. Hydrometallurgy, 2010, 104: 201–206.
YIN Zhou-lan, DING Zhi-ying, HU Hui-ping, LIU Kui, CHEN Qi-yuan. Dissolution of zinc silicate (hemimorphite) with ammonia-ammonium chloride solution [J]. Hydrometallurgy, 2010, 103: 215–220.
LIU Zhi-yong, LIU Zhi-hong, LI Qi-hou, YANG Tian-zu, ZHANG Xu. Leaching of hemimorphite in NH3-(NH4)2SO4-H2O system and its mechanism [J]. Hydrometallurgy, 2012, 125/126: 137–143.
PETER MAKRESKI, GLIGOR JOVANOVSKI, BRANKO KAITNER, ANDREJA GAJOVIC, TOMISLAV BILJAN. Minerals from Macedonia X VIII. Vibrational spectra of some sorosilicates [J]. Vibrational Spectroscopy, 2007, 44: 162–170.
ZHANG Q, MO W, WANG Z. Structure properties of nanosilica prepared by precipitation [J]. Bulletin of the Chinese Ceramic Society, 2005, 24: 118–121.
FARMER V C. The infrared spectra of minerals [M]. Beijing: Science Press, 1982: 292–293.
CASEY W H, HOCHELLA jr M F, WESTRICH H R. The surface chemistry of manganiferous silicate minerals as inferred from experiments on tephroite (Mn2SiO4) [J]. Geochimica Et Cosmochim -ica Acta, 1993, 57(4): 785–793.
LIN Chung-cherng, SHEN Pou-yan. Role of screw axes in dissolution of willemite [J]. Geochimica et Cosmochimica Acta, 1993, 57(8): 1649–1655.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(511340071) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Li, Qx., Chen, Qy. & Hu, Hp. Dissolution mechanism and solubility of hemimorphite in NH3-(NH4)2SO4-H2O system at 298.15 K. J. Cent. South Univ. 21, 884–890 (2014). https://doi.org/10.1007/s11771-014-2014-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-014-2014-4