Abstract
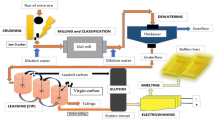
A two-step leaching method in combination of acid and ethylene diaminetetraacetic acid disodium (EDTA-Na2) was applied to extract metals such as Cd, Cu, Fe, Pb and Zn from a zinc smelting slag. The results show that the extraction rates of Cd, Cu, Fe and Zn in slag reach 88.3%, 54.1%, 69.6% and 54.7%, respectively, while the extraction rate of Pb is only 0.05% leached with 1.25 mol/L sulfuric acid under the conditions of the ratio of slag to liquid of 100 g/L, 65 °C and 120 r/min for 2 h. However, Pb extraction rate from 1.25 mol/L sulfuric acid leached residue reaches as high as 66.5% by using 0.1 mol/L EDTA-Na2 solution. The results indicate that two-step sequential extraction procedure combining 1.25 mol/L sulfuric acid and 0.1 mol/L EDTA-Na2 solution can extensively extract Cd, Cu, Fe, Pb and Zn from zinc smelting slag.
Similar content being viewed by others
References
PIATAK N M, SEAL R R. Mineralogy and the release of trace elements from slag from the Hegeler Zinc smelter, Illinois (USA) [J]. Applied Geochemistry, 2010, 25(2): 302–320.
SONKE J E, SIVERY Y, VIERS J, FREYDIER R, DEIONGHE L, ANDRÉ L, AGGARWAL J K, FONTAN F, DUPRÉ B. Historical variations in the isotopic composition of atmospheric zinc deposition from a zinc smelter [J]. Chemical Geology, 2008, 252(3/4): 145–157.
KUL M, TOPKAYA Y. Recovery of germanium and other valuable metals from zinc plant residues [J]. Hydrometallurgy, 2008, 92(3/4): 87–94.
SAFARZADEH M S, MORADKHANI D, ILKHCHI M O. Kinetics of sulfuric acid leaching of cadmium from Cd-Ni zinc plant residues [J]. Journal of Hazardous Materials, 2009, 163(2/3): 880–890.
ARSLANA C, ARSLANB F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags [J]. Hydrometallurgy, 2002, 67(1/2/3): 1–7.
SAFARZADEH M S, MORADKHANI D, ILKHCHI M O, GOLSHAN N H. Determination of the optimum conditions for the leaching of Cd-Ni residues from electrolytic zinc plant using statistical design of experiments [J]. Separation Purification Technology, 2008, 58(3): 367–376.
GOUVEA L R, MORAIS C A. Recovery of zinc and cadmium from industrial waste by leaching/cementation [J]. Minerals Engineering, 2007, 20(9): 956–958.
ZHANG Yang, MAN Rui-lin, NI Wang-dong, WANG Hui. Selective leaching of base metals from copper smelter slag [J]. Hydrometallurgy, 2010, 103(1/2/3/4): 25–29.
MORALES A, CRUELLS M, ROCA A, BERGÓ R. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization [J]. Hydrometallurgy, 2010, 105(1/2): 148–154.
BESE A V, BORULU N, COPUR M, COLAK S, ATA O N. Optimization of dissolution of metals from Waelz sintering waste (WSW) by hydrochloric acid solutions [J]. Chemical Engineering Journal, 2010, 162(2): 718–722.
GUTIÉRREZ R Z, LAPIDUS G T, MORALES R D. Pressure leaching of a lead-zinc-silver concentrate with nitric acid at moderate temperatures between 130 and 170 °C [J]. Hydrometallurgy, 2010, 104(1): 8–13.
JHA M K, KUMAR V, SINGH R J. Review of hydrometallurgical recovery of zinc from industrial wastes [J]. Resource, Conservation and Recycling, 2001, 33(1): 1–22.
FARAHMAND F, MORADKHANI D, SAFARZADEH M S, ASHCHI F R. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology [J]. Hydrometallurgy, 2009, 95(3/4): 316–324.
SENANAYAKEI G. Review of theory and practice of measuring proton activity and pH in concentrated chloride solutions and application to oxide leaching [J]. Minerals Engineering, 2007, 20(7): 634–645.
TURAN M D, ALTUNDOGAN H S, TQMEN F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1/2/3/4): 169–176.
RUSEN A, SUNKAR A S, TOPKAYA Y A. Zinc and lead extraction from Çinkur leach residues by using hydrometallurgical method [J]. Hydrometallurgy, 2008, 93(1/2): 45–50.
PARK Y J. Stabilization of a chlorine-rich fly ash by colloidal silica solution [J]. Journal of Hazardous Materials, 2009, 162(2/3): 819–822.
PALMA L D, FERRANTELLI MERLI P, BIANCIFIORI C F. Recovery of EDTA and metal precipitation from soil flushing solutions [J]. Journal of Hazardous Materials B, 2003, 103(1/2): 153–168.
ZENG Q R, SAUVE S, ALLEN H E, HENDERSHOT W H. Recycling EDTA solutions used to remediate metal-polluted soils [J]. Environmental Pollution, 2005, 133(2): 225–231.
RUMBALL J A, RICHMOND G D. Measurement of oxidation in a base metal flotation circuit by selective leaching with EDTA [J]. International Journal of Mineral Processing, 1996, 48(1/2): 1–20.
GREET C, SMART R S C. Diagnostic leaching of galena and its oxidation products with EDTA [J]. Minerals Engineering, 2002, 15(7): 515–522.
FEDIE K K, EKBERG C, SKARNEMARK G, STEENARI B M. Removal of hazardous metals from MSW fly ash-An evaluation of ash leaching methods [J]. Journal Hazardous Materials, 2010, 173(1/2/3): 310–317.
GUO Zhao-hui, PAN Feng-kai, XIAO Xi-yuan, ZHANG Long, JIANG Kai-qi. Optimization of brine leaching of metals from hydrometallurgical residue [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 1–6.
NOWACK B, SCHULIN R, ROBINSON B H. Critical assessment of chelant-enhanced metal phytoextraction [J]. Environmental Science Technology, 2006, 40(17): 5225–5232.
FINŽGAR N, LEŠTAN D. Heap leaching of Pb and Zn contaminated soil using ozone/UV treatment of EDTA extractants [J]. Chemosphere, 2006, 63(10): 1736–1743.
FINŽGAR N, LEŠTAN D. Multi-step leaching of Pb and Zn contaminated soils with EDTA [J]. Chemosphere, 2007, 66(5): 824–832.
NÚŇEZ-LÓPEZ R A, MEAS Y, GAMA S C, BORGES R O, OLGUÍN E J. Leaching of lead by ammonium salts and EDTA from Salvinia minima biomass produced during aquatic phytoremediation [J]. Journal Hazardous Materials, 2008, 154(1/2/3): 623–632.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2011SK3262) supported by Science and Technology Program of Hunan Province, China
Rights and permissions
About this article
Cite this article
Jiang, Kq., Guo, Zh., Xiao, Xy. et al. Extraction of metals from a zinc smelting slag using two-step procedure combining acid and ethylene diaminetetraacetic acid disodium. J. Cent. South Univ. 19, 1808–1812 (2012). https://doi.org/10.1007/s11771-012-1212-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1212-1