Abstract
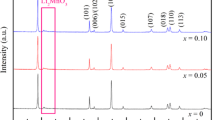
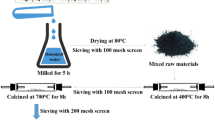
The synthesis, structure and performance of Li2Mg0.15Mn0.4Co0.45SiO4/C cathode material were studied. The Li2Mg0.15Mn0.4Co0.45SiO4/C solid solution with orthorhombic unit cell (space group Pmn21) was synthesized successfully by combination of wet process and solid-state reaction at high temperature, and its electrochemical performance was investigated primarily. Li2Mg0.15Mn0.4Co0.45SiO4/C composite materials deliver a charge capacity of 302 mA·h/g and a discharge capacity of 171 mA·h/g in the first cycle. The discharge capacity is stabilized at about 100 mA·h/g after 10 cycles at a current density of 10 mA/g in the voltage of 1.5–4.8 V vs Li/Li+. The results show that Mg-substitution for the Co ions in Li2Mn0.4Co0.6SiO4 improves the stabilization of initial structure and the electrochemical performance.
Similar content being viewed by others
References
KOYAMA Y, MAKIMURA Y, TANAKA I, ADACHI H, OHZUKU T. Systematic research on insertion materials based on superlattice models in a phase triangle of LiCoO2-LiNiO2-LiMnO2. I. First-principles calculation on electronic and crystal structures, phase stability and new LiNi1/2Mn1/2O2 material [J]. J Electrochem Society A, 2004, 151(9): 1499–1506.
CHANG Zhao-rong, CHEN Zhong-jun, WU Feng, TANG Hong-wei, ZHU Zhi-hong, YUAN Xiao-zi, WANG Hai-jiang. Synthesis and characterization of high-density non-spherical Li(Ni1/3Co1/3Mn1/3)O2 cathode material for lithium ion batteries by two-step drying method [J]. Electrochimica Acta, 2008, 53: 5927–5933.
CHUNG Kyung-yoon, KIM Kwang-bum. Investigations into capacity fading as a result of a Jahn-Teller distortion in 4 V LiMn2O4 thin film electrodes [J]. Electrochimica Acta, 2004, 4(20): 3327–3337.
PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144: 1188–1194.
AMINE K, YASUDA H, YAMACHI M. Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries [J]. Electrochem Solid-State Lett, 2000, 3: 178–179.
LI Guo-hua, HIDETO AZUMA, MASAYUKI TOHDA. LiMnPO4 as the cathode for lithium batteries [J]. Electrochem Solid-State Lett A, 2002, 5: 135–137.
ZHANG Z R, GONG Z L, YANG Yong. Electrochemical performance and surface properties of bare and TiO2-Coated cathode materials in lithium-ion batteries [J]. J Phys Chem B, 2004, 108: 17546–17552.
LI Yi-xiao, GONG Zheng-liang, YANG Yong. Synthesis and characterization of Li2MnSiO4/C nanocomposite cathode material for lithium ion batteries [J]. J of Power Sources, 2007, 174: 528–532.
NYTEN A, ABOUIMRANE A, ARMAND M, GUSTAFFSON T, THOMAS J O. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material [J]. Electrochem Commun, 2005, 7: 156–160.
DOMINKO R, BELE M, GABERSCEK M, MEDEN A, REMSKAR M, JAMNIK J. Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials [J]. Electrochem Commun, 2006, 8: 217–222.
GONG Zheng-liang, LI Yi-xiao, YONG Yong. Synthesis and characterization of Li2MnxFe1−x SiO4 as a cathode material for lithium-ion batteries [J]. Electrochem Solid-State Letters A, 2006, 9: 542–544.
KOKALJ A, DOMINKO R, MALI G, MEDEN A, GABERSCEK M, JAMNIK J. Beyond one-electron reaction in Li cathode materials: Designing Li2MnxFe1−x SiO4, chem. [J]. Mater, 2007, 19: 3633–3640.
NESS C, DELOBEL B, ARMSTRONG A R, BRUCE P G. The lithium intercalation compound Li2CoSiO4 and its behaviour as a positive electrode for lithium batteries [J]. Chem Commun (Camb), 2007, 46: 4890–4892.
GONG Zheng-liang, LI Yi-xiao, YONG Yong. Synthesis and electrochemical performance of Li2CoSiO4 as cathode material for lithium ion batteries [J]. J of Power Sources, 2007, 174: 524–527.
WU Shun-qing, ZHU Zi-zhong, YANG Yong, HOU Zhu-feng. Effects of Na-substitution on structural and electronic properties of Li2CoSiO4 cathode material [J]. Trans Nonferrous Met Soc China, 2009, 19: 182–186.
ZAGHIB K, AIT SALAH A, RAVET N, MAUGER A, GENDRON F, JULIEN C M. Structural, magnetic and electrochemical properties of lithium iron orthosilicate [J]. J Power Sources, 2006, 160: 1381–1386.
ARROYO de DOMPABLO M E, ARMAND M, TARASCON J M, AMADOR U. On-demand design of polyoxianionic cathode materials based on electronegativity correlations: An exploration of the Li2MSiO4 system (M=Fe, Mn, Co, Ni) [J]. Electrochem Commun, 2006, 8: 1292–1298.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(10B054) supported by Scientific Research Fund of Hunan Provincial Education Department, China; Projects(2011GK2002, 2011FJ3160) supported by the Planned Science and Technology Program of Hunan Province, China
Rights and permissions
About this article
Cite this article
Hu, Cy., Guo, J., Li, Sj. et al. Synthesis and electrochemical performance of Li2Mg0.15Mn0.4Co0.45SiO4/C cathode material for lithium ion batteries. J. Cent. South Univ. 19, 1791–1795 (2012). https://doi.org/10.1007/s11771-012-1209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1209-9