Abstract
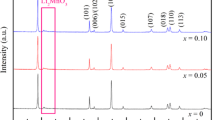
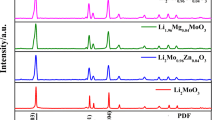
Li2Mn0.98M0.02SiO4 (M = Mg, Ni, Cr) cathode material for lithium-ion batteries was synthesized by traditional solid-phase doping method used Li2CO3, MnCO3, (C2H5O)4Si, and MgC2O4·2H2O (or NiC2O4·2H2O or C2O3) as staring materials. The suitable calcination temperature (700 °C) was obtained by TG-DTA curve. The influence of different doping amount on the crystal structure, micromorphology, and electrochemical properties of Li2MnSiO4 was studied by XRD, SEM, and electrochemical performance measurement. The XRD patterns indicate that the Li2MnSiO4 crystallized in an orthorhombic structure with a space group of Pmn21. It can be seen from SEM images that Mg doping has no effect on the micromorphology, Cr doping can refine the powder, and the particle size is about 200–800 nm. The electrochemical performance measurement demonstrates that the Li2Mn0.98Cr0.02SiO4 shows the best electrochemical performance with an initial discharge capacity of 29.8 mAh g−1 at 0.1 C, and the discharge capacity retention rate after 70 cycles is 20.1%.






Similar content being viewed by others
References
Li J, Luo S, Ding X, Wang Q, He P (2018) Three-dimensional honeycomb-structural LiAlO2-modified LiMnPO4 composite with superior high rate capability as Li-ion battery cathodes. ACS Appl Mater Inter 10(13):10786–10795. https://doi.org/10.1021/acsami.7b17597
Liu C, Luo S, Huang H, Wang Z, Wang Q, Zhang Y, Liu Y, Zhai Y, Wang Z (2018) Potassium vanadate K0.23V2O5 as anode materials for lithium-ion and potassium-ion batteries. J Power Sources 389:77–83. https://doi.org/10.1016/j.jpowsour.2018.04.014
Liu H, Luo S, Yan S, Wang Q, Hu D, Wang Y, Feng J, Yi T (2019) High-performance α-Fe2O3/C composite anodes for lithium-ion batteries synthesized by hydrothermal carbonization glucose method used pickled iron oxide red as raw material. Compos Part B Eng 164:576–582. https://doi.org/10.1016/j.compositesb.2019.01.084
Effect of structure on the Fe3 + / Fe2 + redox couple in iron phosphates
Arroyo-de Dompablo ME, Armand M, Tarascon JM, Amador U (2006) On-demand design of polyoxianionic cathode materials based on electronegativity correlations: An exploration of the Li2MSiO4 system (M=Fe, Mn, Co, Ni). Electrochem Commun 8(8):1292–1298. https://doi.org/10.1016/j.elecom.2006.06.003
Dominko R, Bele M, Gaberšček M, Meden A, Remškar M, Jamnik J (2006) Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials. Electrochem Commun 8(2):217–222. https://doi.org/10.1016/j.elecom.2005.11.010
Qiu S, Pu X, Ai X, Yang H, Chen Z, Cao Y (2018) Template synthesis of mesoporous Li2MnSiO4@C composite with improved lithium storage properties. Electrochim Acta 291:124–131. https://doi.org/10.1016/j.electacta.2018.08.146
Shree Kesavan K, Michael MS, Prabaharan SRS (2019) Facile electrochemical activity of monoclinic Li2MnSiO4 as potential cathode for Li-ion batteries. ACS Appl Mater Inter 11(32):28868–28877. https://doi.org/10.1021/acsami.9b08213
Yan X, Hou Y, Huang Y, Zheng S, Shi Z, Tao X (2019) Effects of Ga, Ge and As modification on structural, electrochemical and electronic properties of Li2MnSiO4. J Electrochem Soc 166(15):A3874–A3880. https://doi.org/10.1149/2.1321915jes
Dominko R, Bele M, Kokalj A, Gaberscek M, Jamnik J (2007) Li2MnSiO4 as a potential Li-battery cathode material. J Power Sources 174(2):457–461. https://doi.org/10.1016/j.jpowsour.2007.06.188
Zhu H, Deng W, Chen L, Zhang S (2019) Nitrogen doped carbon layer of Li2MnSiO4 with enhanced electrochemical performance for lithium ion batteries. Electrochim Acta 295:956–965. https://doi.org/10.1016/j.electacta.2018.11.133
Wang C, Xu Y, Sun X, Zhang B, Chen Y, He S (2018) Enhanced electrochemical properties of F-doped Li2MnSiO4/C for lithium ion batteries. J Power Sources 378:345–352. https://doi.org/10.1016/j.jpowsour.2017.12.004
Modification and deterioration mechanism of lithium manganese silicate as cathode material for lithium-ion batteries. https://doi.org/10.7521/j.issn.0454-5648.2013.10.14
Effect of Ni substitution on structural stability, micromorphology, and electrochemical performance of Li2MnSiO4/C cathode materials
Cheng H, Zhao S, Wu X, Zhao J, Wei L, Nan C (2018) Synthesis and structural stability of Cr-doped Li 2 MnSiO 4/C cathode materials by solid-state method. Appl Surf Sci 433:1067–1074. https://doi.org/10.1016/j.apsusc.2017.10.045
Spectrochim Acta A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2014.02.095
Wei Y, Wang LJ, Yan J et al (2011) Calcination temperature effects on the electrochemical performance ofLi2MnSiO4/C cathode material for Lithium ion batteries. Acta Phys -Chim Sin 27(11):2587–2592
Duncan H, Kondamreddy A, Mercier PHJ, Le Page Y, Abu-Lebdeh Y, Couillard M, Whitfield PS, Davidson IJ (2011) Novel Pn polymorph for Li2MnSiO4 and its electrochemical activity as a cathode material in Li-ion batteries. Chem Mater 23(24):5446–5456. https://doi.org/10.1021/cm202793j
Liu C, Luo S, Huang H, Zhai Y, Wang Z (2019) Layered potassium-deficient P2- and P3-type cathode materials KxMnO2 for K-ion batteries. Chem Eng J 356:53–59. https://doi.org/10.1016/j.cej.2018.09.012
Zhou LZ, Xu QJ, Liu MS, Jin X (2013) Novel solid-state preparation and electrochemical properties of Li1.13[Ni0.2Co0.2Mn0.47]O2 material with a high capacity by acetate precursor for Li-ion batteries. Solid State Ionics 249-250:134–138. https://doi.org/10.1016/j.ssi.2013.07.024
Arsentev M, Hammouri M, Kovalko N, Kalinina M, Petrov A (2017) First principles study of the electrochemical properties of Mg-substituted Li2MnSiO4. Comp Mater Sci 140:181–188. https://doi.org/10.1016/j.commatsci.2017.08.045
Saracibar A, Wang Z, Carroll KJ, Meng YS, Dompablo MEA (2015) New insights into the electrochemical performance of Li2MnSiO4: effect of cationic substitutions. J Mater Chem A 3(11):6004–6011. https://doi.org/10.1039/C4TA03367A
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 51874079, 51674068, 51804035); the Natural Science Foundation of Hebei Province (No. E2018501091); the Training Foundation for Scientific Research of Talents Project, Hebei Province (No. A2016005004); the Fundamental Research Funds for the Central Universities (Nos. N172302001, N182312007, N182306001, N2023040); and the Hebei Province Key Research and Development Plan Project (No. 19211302D).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Zhan, Y., Luo, Sh. et al. Preparation and electrochemical properties of cationic substitution Li2Mn0.98M0.02SiO4 (M = Mg, Ni, Cr) as cathode material for lithium-ion batteries. Ionics 26, 3769–3775 (2020). https://doi.org/10.1007/s11581-020-03570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03570-0