Abstract
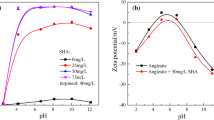
Two reagents including salicylhydroxamic acid (SHA) and tributyl phosphate (TBP) were tested as collectors either separately or together for electro-flotation of fine cassiterite (<10 μm). Subsequently, the flotation mechanism of the fine cassiterite was investigated by adsorbance determination, electrophoretic mobility measurements and Fourier transform infra-red (FT-IR) spectrum checking. Results of the flotation experiments show that with SHA as a collector, the collecting performance is remarkably impacted by the pulp pH value as the floatability of cassiterite varies sharply when the pH changes, and flotation with SHA gives distinct maximum at about pH 6.5. Additionally, the floatability of cassiterite is determined by using SHA and TBP as collectors. The range of pulp pH for good floatability is broadened in the presence of TBP as auxiliary collector, and the utilization of TBP improves the recovery of cassiterite modestly. Moreover, the optimum pH value for cassiterite flotation is associated with adsorbance. The results of FT-IR spectrum and the electrophoretic mobility measurements indicate that the adsorption interaction between the collectors and the cassiterite is dominantly a kind of chemical bonding in the form of one or two cycle chelate rings due to the coordination of carbonyl group, hydroxamate and P=O group to the metal tin atoms, where the oxygen atoms contained in carbonyl group, hydroxamate and P=O group of the polar groups have the stereo conditions to form five-membered rings. In addition, the adsorption interactions of SHA and TBP on the surfaces of cassiterite are also dominated by means of hydrogen bonds.
Similar content being viewed by others
References
GEORGE P, NGUYEN A V, JAMESON G J. Assessment of true flotation and entrainment in the flotation of submicron particles by fine bubbles [J]. Minerals Engineering, 2004, 17(7/8): 847–853.
WATERS K E, HADLER K, CILLIERS J J. The flotation of fine particles using charged microbubbles [J]. Minerals Engineering, 2008, 21(12/13/14): 918–923.
MIETTINEN T, RALSTON J, FORNASIERO D. The limits of fine particle flotation [J]. Minerals Engineering, 2010, 23(5): 420–437.
GONTIJO C D, FORNASIERO D, RALSTON J. The limits of fine and coarse particle flotation [J]. Canadian Journal of Chemical Engineering, 2007, 85(5): 739–747.
CILEK E C, UMUCU Y. A statistical model for gangue entrainment into froths in flotation of sulphide ores [J]. Minerals Engineering, 2001, 14(9): 1055–1066.
SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 205(1/2): 47–59.
JIANG Y R, ZHAO B N, ZHOU X H, ZHOU L Y. Flotation of diaspore and aluminosilicate minerals applying novel carboxyl hydroxamic acids as collector [J]. Hydrometallurgy, 2010, 104(1): 112–118.
HU Y, LIU X, XU Z H. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite [J]. Minerals Engineering, 2003, 16(3): 219–227.
WU X Q, ZHU J G. Selective flotation of cassiterite with benzohydroxamic acid [J]. Minerals Engineering, 2006, 19(14): 1410–1417.
FUERSTENAU D W, PRADIP. Zeta potentials in the flotation of oxide and silicate minerals [J]. Advances in Colloid and Interface Science, 2005, 114: 9–26.
CHEN G L, TAO D, REN H, JI F F, QIAO J K. An investigation of niobite flotation with octyl diphosphonic acid as collector [J]. International Journal of Mineral Processing, 2005, 76(1/2): 111–122.
SOMASUNDARAN P, UNJAPPU J T K. In-situ investigation of adsorbed surfactants and polymers on solids in solution [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1989, 37: 245–268.
OZCAN O, BULUTCU A N. Electrokinetic, infrared and flotation studies of scheelite and calcite with oxine, alkyl oxine, oleoyl sarcosine and quebracho [J]. International Journal of Mineral Processing, 1993, 39(3/4): 275–290.
HU Y H, XU Z H. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation [J]. International Journal of Mineral Processing, 2003, 72(1/4): 87–94.
LIMA R M F, BRANDAO P R G, PERES A E C. The infrared spectra of amine collectors used in the flotation of iron ores [J]. Minerals Engineering, 2005, 18(2): 267–273.
VIDYADHAR A, RAO K H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system [J]. Journal of Colloid and Interface Science, 2007, 306(2): 195–204.
HU Y, WANG D, XU Z. A study of interactions and flotation of wolframite with octyl hydroxamate [J]. Minerals Engineering, 1997, 10(6): 623–633.
ASSIS S M, MONTENEGRO L C M, PERES A E C. Utilisation of hydroxamates in minerals froth flotation original research article [J]. Minerals Engineering, 1996, 9(1): 103–114.
YOON R H, NAGARAJ D R, WANG S S, HILDEBRAND T M. Benefication of kaolin clay by froth flotation using hydroxamate collectors [J]. Minerals Engineering, 1992, 5(3/5): 457–467.
ZHU D, ZHU Y S. Research on surface electric property of cassiterite [J]. Non-ferrous Mining and Metallurgy, 1994(1): 19–22. (in Chinese)
SHI D M. Cassiterite flotation and surface electric property [J]. Journal of Kunming University of Science and Technology: Science and Technology, 1987, (3): 38–45. (in Chinese)
THOMAS L C, CHITTENDE R A. Characteristic infrared absorption frequencies of organophosphorus compounds: I. The phosphoryl (P-O) group [J]. Spectrochimica Acta, 1964, 20(3): 467–487.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(50774094) supported by the National Natural Science Foundation of China; Project(2010CB630905) supported by the National Basic Research Program of China
Rights and permissions
About this article
Cite this article
Qin, Wq., Ren, Ly., Xu, Yb. et al. Adsorption mechanism of mixed salicylhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system. J. Cent. South Univ. Technol. 19, 1711–1717 (2012). https://doi.org/10.1007/s11771-012-1197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1197-9