Abstract
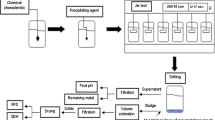
The solid sodium hydroxide neutralized acidic As-containing wastewater till pH value was 6. Green copper arsenite was prepared after copper sulfate was added into the neutralized wastewater when the molar ratio of Cu to As was 2:1 and pH value of the neutralized wastewater was adjusted to 8.0 by sodium hydroxide. The arsenious acid solution and red residue were produced after copper arsenite mixed with water according to the ratio of liquid to solid of 4:1 and copper arsenite was reduced by SO2 at 60 °C for 1 h. The white powder was gained after the arsenious acid solution was evaporated and cooled. Copper sulfate solution was obtained after the red residue was leached by H2SO4 solution under the action of air. The results show that red residue is Cu3(SO3)2·2H2O and the white powder is As2O3. The leaching rate of Cu reaches 99.00% when the leaching time is 1.5 h, molar ratio of H2SO4 to Cu is 1.70, H2SO4 concentration is 24% and the leaching temperature is 80 °C. The direct recovery rate of copper sulfate is 79.11% and the content of CuSO4·5H2O is up to 98.33% in the product after evaporating and cooling the copper sulfate solution.
Similar content being viewed by others
References
DUTRÉ V, VANDECASTEELE C. Solidification/stabilisation of hazardous arsenic containing waste from a copper refining process [J]. Journal of Hazardous Materials, 1995, 40(1): 55–68.
LEIST M, CASEY R J, CARIDI D. The management of arsenic wastes: Problems and prospects [J]. Journal of Hazardous Materials, 2000, 76(1): 125–138.
RIOS-ARANA J V, WALSH E J, GARDEA-TORRESDEY J L. Assessment of arsenic and heavy metal concentrations in water and sediments of the Rio Grande at El Paso-Juarez metroplex region [J]. Environment International, 2004, 29(7): 957–971.
HELSEN L, van den BULCK E. Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste with detailed analyses of thermochemical conversion processes [J]. Environmental pollution, 2005, 134(2): 301–314.
RIVEROS G, UTIGARD T A. Disposal of arsenic in copper discharge slags [J]. Journal of Hazardous Materials, 2000, 77(1/3): 241–252.
ALEKSAN D, WITKIEWICZ K, BAL W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis [J]. Toxicology Letters, 2006, 162(1): 29–42.
SHIH C J, LIN C F. Arsenic contaminated site at an abandoned copper smelter plant: Waste characterization and solidification/ stabilization treatment [J]. Chemosphere, 2003, 53(7): 691–703.
SONG S, VALDIVIESO A L, CAMPOS D J H. Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite [J]. Water Research, 2006, 40(2): 364–372.
SHAABAN E R. Optical characterization of arsenic sulfide semiconducting glass films using the transmittance measurements [J]. Materials Chemistry and Physics, 2006, 100(2/3): 411–417.
JAY J A, BLUTE N K, HEMOND H F. Arsenic-sulfides confound anion exchange resin exchange resin speciation of aqueous arsenic [J]. Water Research, 2004, 38(5): 1155–1158.
MIHAJLOVIC I, STRBAC N, ZIVKOVIC Z, KOVACEVIC R, STEHERNIK M. A potential method for arsenic removal from copper concentrates [J]. Minerals Engineering, 2007, 20(1): 26–33.
LIU Jian-chao, HE Hong-wu, FENG Xin-min. Direction of chemical pesticide development green chemical pesticides [J]. Pesticides, 2005, 44(1): 1–3.
GUAN Yu-jiang, CHEN Liu-chen. Study on the treatment of wastewater from sulphuric acid production by lime PFS process [J]. Environmental Protection of Chemical Industry, 1999, 19(6): 328–335. (in Chinese)
CHAN B K C, DUDENEY A W L. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates [J]. Minerals Engineering, 2008, 21(4): 272–278.
NISHIMURA T, ITOH C T, TOZAWA K. Stability and solubilities of metal arsenates and arsenates in water and effect of sulfate and carbonate ions on their solubilities[C]// REDDY R G, HENDRIX J L, QUENEAU P B. Arsenic Fundamentals and Applications. Warrendale, PA: TMS, 1988: 77–97.
ZHENG Ya-jie, WANG Yong, ZHAO Pan-feng. A method of producing arsenite copper and arsenate copper from waste acid contained As: CN, 200610031980.7 [P]. 2006-10-25. (in Chinese)
ZHENG Ya-jie, XIAO Fa-xin, WANG Yong. Preparation and application of copper arsenite: CN, 200610031980.7 [P]. 2006-07-19. (in Chinese)
WANG Yong, ZHAO Pan-feng, ZHENG Ya-jie. Preparation of copper arsenite from waste acid containing arsenic and its application in copper electrolyte purification [J]. Journal of Central South University: Science and Technology, 2007, 38(6): 1115–1120. (in Chinese)
KHANDAKER N R, TETER D M, KRUMHANSL J L. Arsenic removal in conjunction with lime softening: US 6802980B1 [P]. 2004-12-12.
ZHENG Ya-jie, LUO Yuan, WANG Yong. Method of preparing arsenic trioxide from As-containing wastewater: CN, 200710035704.2 [P]. 2007-09-07. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Yj., Wang, Y., Xiao, Fx. et al. Recovery of copper sulfate after treating As-containing wastewater by precipitation method. J. Cent. South Univ. Technol. 16, 242–246 (2009). https://doi.org/10.1007/s11771-009-0041-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-009-0041-3