Abstract
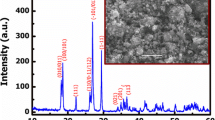
The capacity intermittent titration technique (CITT) was developed based on the ratio of potentio-charge capacity to galvano-charge capacity (RPG) method, to continuously determine the solid diffusion coefficient (D) of the intercalary species within insertion-host materials with a small voltage region. The linear equations of D vs the value of ratio of the potentio-charge capacity to the galvano-charge capacity (q) were given. By the CITT technique, the Li+ solid diffusion coefficients within LiMn2O4 at different voltages were determined. The results show that the values of D varied from 3.447 × 10−9 to 7.60 × 10−11 cm2/s in the voltage range of charge from 3.3 to 4.3 V as a function of voltage with “W” shape.
Similar content being viewed by others
References
Arora P, White R E. Capacity fade mechanisms and side reactions in lithium-ion batteries [J]. J Electrochem Soc, 1998, 145: 3647–3667.
Uchina T, Marikaua Y, Ikuta H, et al. Chemical diffusion coefficient of lithium in carbon fiber[J]. J Electrochem Soc, 1996, 143: 2606–2610.
Weppner W, Huggins R A. Determination of the kinetic parameters of mixed-conducting electrodes and application to the systerm Li3Sb [J]. J Electrochem Soc, 1977, 124: 1569–1576.
Yu P, Popov B N, Ritter J A, et al. Determination of the lithium ion diffusion coefficient in graphite [J]. J Electrochem Soc, 1999, 146: 8–14.
Zhang D, Popov B N, White R E. Electrochemical investigation of CrO2.65 doped LiMn2O4 as a cathode material for lithium-ion batteries [J]. J Power Sources, 1998, 76: 81–90.
Deiss E. Spurious potential dependence of diffusion coefficients in Li+ insertion electrode measured with PITT[J]. Electrochimica Acta, 2002, 47: 4027–4034.
Macdonald D D. Transient Techniques in Electrochemistry[M]. New York: Plenum Press, 1977.
Wang Q, Li H, Huang X L, et al. Determination of chemical diffusion coefficient of lithium ion in graphitized mesocarbon microbeads with potential relaxation technique[J]. J Electrochem Soc, 2001, 48: A737-A741.
Tang X C, He L P, Chen Z Z, et al. Determination of the Li+ diffusion coefficient in graphite by the method of the ratio of potentio-charge capacity to galvanocharge capacity[J]. Acta Phys Chim Sin, 2002, 18: 705–709. (in Chinese)
Tang X C, Pan C Y, He L P, et al. A novel technique based on the ratio of potentio-charge capacity to galvano-charge capacity (RPG) for determination of the diffusion coefficient of intercalary species within insertion-host materials: theories and experiments [J]. Electrochimica Acta, 2004, 49: 3113–3119.
Tang X C, Yang Y P, Li L Q, et al. Electrochemical properties of spinel LiMn2O4 prepared by thermo-decomposition of LiMn2L(Ac)2(L: citric acid radical) [J]. Chinese J Nonferrous Metals, 2004, 14: 871–876. (in Chinese)
Huang Y D, Li J, Jia D Z. Preparation and characterization of positive electrode material LiMn2O4 for lithium ion battery by low heating solid state coordination method[J]. Chinese J Inorg Chem, 2004, 7: 835–840. (in Chinese)
Hjelm A K, Lindberg G. Experimental and theoretical analysis of LiMn2O4 cathodes for use in rechargeable lithium batteries by electrochemical impedance spectroscopy (EIS)[J]. Electrochimica Acta, 2002, 47: 1747–1759.
Cao F, Prakash J. A comparative electrochemical study of LiMn2O4 spinel thin-film and porous laminate [J]. Electrochimica Acta, 2002, 47: 1607–1613.
Darling R, Newman J. Dynamic monte carlo simulations of diffusion in LiyMn2O4 [J]. J Electrochem Soc, 1999, 146: 3765–3772.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project (20406024) supported by the National Natural Science Foundation of China: project (76600) supported by the Postdoctoral Science Foundation of Central South University
Rights and permissions
About this article
Cite this article
Tang, Xc., Huang, By. & He, Yh. Determination of Li+ solid diffusion coefficient in LiMn2O4 by CITT. J Cent. South Univ. Technol. 12 (Suppl 1), 1–4 (2005). https://doi.org/10.1007/s11771-005-0360-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-005-0360-y