Abstract
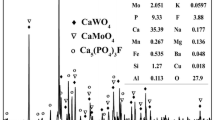
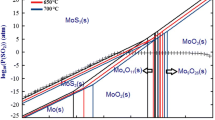
A new technology of treating molybdenum residues by simultaneous ultrafine milling and alkali leaching was put forward to recover molybdenum from metallurgical residues. The effects of residue size, milling time, solid content, n(Na2CO3)/n(Mo) and slurry pH value on molybdenum leaching rate were investigated. The results indicate that a simpler process, lower slurry temperature, 50% shorter treating time, 60% decrease of Na2CO3 content and 15% increase of molybdenum leaching rate can be obtained by the new technology compared with the traditional process. The leaching kinetic equation was determined, and calculation of active energy (E=56.2 kJ/mol) shows that the leaching process of molybdenum residues by simultaneous ultrafine milling and alkali leaching is controlled by chemical reaction. Potential exists for the new process to form the basis for an economically viable, environmentally friendly process to recover valuable elements from residues.
Similar content being viewed by others
References
Stehr N. Recent development in stirred ball milling[J]. Int J Miner Process, 1988, 22: 431–444.
Warris C J, McCormick P G. Mechanochemical processing of refractory pyrite[J]. Miner Eng, 1997, 10(10): 1119–1125.
Zheng J, Somasundaran P. Power consumption of stirred media mills[J]. Miner Metal Process, 1995, (1): 34–40.
Davis S B, Dawson M F. A laboratory study of attrition grinding[J]. J South African Institute of Mining and Metallurgy, 1989, 89(8): 231–241.
Kerr M C, Reed J S. Comparative grinding kinetics and grinding energy during ball milling and attrition[J]. American Ceramics Society Bulletin, 1992, 71(2): 1809–1816.
Gao M, Forssberg E. A study on the effects of parameters in stirred ball milling[J]. Int J Miner Process, 1993, 37: 45–49.
Queneau P B. The kinetics of the dissolution of scheelite in alkaline aqueous solution[J]. Trans AIME, 1969, 254(11): 2451–2453.
Sohn H Y. Rate process of extractive metallurgy[M]. New York: Plenum Press, 1979: 49–51.
Author information
Authors and Affiliations
Additional information
Foundation item: The Excellent Doctoral Dissertation Foundation of Human Province(No. 200114)
Biography of the first author: YANG Hua-ming, associate professor, doctor, born in Oct. 1968, majoring in mineral processing, nanometer materials and functional ceramics.
Rights and permissions
About this article
Cite this article
Yang, Hm., Hu, Yh. & Qiu, Gz. Recovery of molybdenum from residues by simultaneous ultrafine milling and alkali leaching. J Cent. South Univ. Technol. 9, 87–90 (2002). https://doi.org/10.1007/s11771-002-0048-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11771-002-0048-5