Abstract
Purpose
Data evaluating cardiovascular disease (CVD) risk by cancer treatment among young women (≤ 40 years) with breast cancer are limited.
Methods
Among 372 five-year breast cancer survivors aged 30–40 years from the Young Women's Breast Cancer Study, we assessed the association of cancer treatments (anthracyclines, trastuzumab, radiation/laterality, endocrine therapy) and excess heart age (difference between predicted 10-year CVD risk as assessed by adapted Framingham Risk Score and chronological age), prevalent elevated excess heart age (≥ 2 years), and worsening excess heart age (change of ≥ 2 excess heart age years) at breast cancer diagnosis and two- and five-year follow-up using multivariable linear and logistic regressions.
Results
Most women had stage I or II (79%), ER + (71%), or PR + (65%) breast cancer. At diagnosis, women had little excess heart age by treatment receipt (range of means = -0.52,0.91 years). Left-sided radiation (β = 2.49,SE = 0.96,p = 0.01) was associated with higher excess heart age at five-year follow-up. For prevalent elevated excess heart age (two-year = 26%;five-year = 27%), women treated with right-sided radiation had increased risk at two-years (OR = 2.17,95%CI = 1.12–4.19), yet at five-years, associations were observed after any radiation (OR = 1.92,95%CI = 1.09–3.41), especially after left-sided (OR = 2.13,95%CI = 1.09–3.41) radiation. No associations were observed between systemic treatments and prevalent elevated excess heart age or any treatments with worsening excess heart age.
Conclusions
Among young breast cancer survivors, radiation, but not other cancer treatments, was associated with elevated excess heart age.
Implications for cancer survivors
CVD risk tools that incorporate cancer treatment, such as radiation, are needed to identify high risk young breast cancer survivors given the long survivorship and long latency of cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With improved survival due to advances in treatment [1], young adults with breast cancer (diagnosed at 40 years of age or younger) are likely to have long periods of survivorship and, thus, may prematurely develop cardiovascular disease resulting from late effects of cancer treatment. Young patients are often diagnosed with more advanced disease and may need more aggressive cancer treatments [2]. Cancer treatment such as anthracyclines, trastuzumab, radiation, and endocrine therapy are associated with cardiotoxicity [3,4,5,6,7]. Data are limited surrounding cardiovascular disease risk for young breast cancer survivors; however, there is extensive research in childhood cancer survivors and adolescent and young adult (AYA) cancer survivors who receive similar treatments that have characterized late cardiotoxicity after cancer treatment [8,9,10,11]. For example, childhood cancer survivors have a 15 times higher risk of developing heart failure and 10 times higher risk of developing coronary artery disease relative to their siblings [9], and AYA cancer survivors (including those with breast cancer) have a two-fold increased risk of developing any cardiovascular disease compared to individuals without cancer [10]. Further, the risk of mortality among AYA cancer survivors who develop cardiovascular disease is 11-fold higher compared to cancer survivors without cardiovascular disease [10]. Mechanisms by which cancer treatments affect the cardiovascular disease system in AYA cancer survivors could be similar, yet clinical relevance to young breast cancer survivors may be limited due to differences in cancer types and treatments, gender, and age.
Current clinical guidelines in the U.S. provide recommendations for screening and monitoring of cardiovascular disease in patients receiving cardiotoxic cancer treatments [12,13,14]. However, recommendations are limited to 1–2 years post breast cancer diagnosis, and younger patients who receive cardiotoxic treatments may be at increased risk for developing cardiovascular disease many years or even decades later. A prior study conducted among young breast cancer survivors demonstrated that cardiovascular disease risk, measured using excess heart age, increased two years after diagnosis among those receiving endocrine therapy [15]. To expand on these findings, the present study was conducted in a larger, multicenter prospective cohort of women diagnosed with breast cancer at age 40 years or younger, and examined associations between cancer treatment and excess heart age at two and five years following diagnosis. Because cardiovascular disease is the leading cause of non-cancer deaths among breast cancer survivors [16, 17], evaluating cardiovascular risk among the youngest survivors, where data are limited, can inform cardiovascular disease prevention and monitoring in long-term follow-up.
Methods
Study cohort
The Young Women’s Breast Cancer Study (YWS) enrolled 1,302 women aged ≤ 40 years and diagnosed with breast cancer between 2006–2016 across 13 academic and community‐based centers in the United States and Canada. YWS was approved by the Institutional Review Board at the Dana‐Farber/Harvard Cancer Center and other participating sites. Eligible participants were identified through rapid case ascertainment or clinic list review within 6 months of their breast cancer diagnosis, and an invitation letter was sent to women to participate. Data from participants included serial surveys (collected every 6 months in the first 3 years and annually thereafter), and clinical data were abstracted from medical record review. Supplemental medical record abstraction was conducted for the present study to obtain blood pressures which were not collected as part of the standard medical record abstraction.
Analytic population
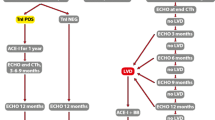
Inclusion criteria for the present analysis were women diagnosed with Stage 0-III breast cancer between the ages 30–40 years, who had follow-up data for at least five years (defined as women who completed either the four-, five-, or six-year survey). We initially excluded women who completed abbreviated surveys (n = 91) or did not have accessible follow-up data in electronic medical records (n = 247). Among patients with available survey and medical record data, we further excluded individuals who had a self-reported a history of cardiovascular disease on the baseline survey (n = 1), were pregnant at the time of survey administration (n = 24), had stage IV disease at diagnosis or who developed a distant or local recurrence of breast cancer within the first 5 years of diagnosis (n = 19), and those missing any heart age variables (i.e., blood pressure, blood pressure medication use, body mass index, history of diabetes, and smoking status) (n = 70). Supplemental Fig. 1 demonstrates a consort diagram for selection of the study cohort.
Study outcome
Our primary outcome was excess heart age calculated at two- and five-years post-diagnosis. Heart age is an adapted version of the Framingham Risk Score, which is a 10-year estimate of cardiovascular disease risk [18] and is a gender-based equation calculated using chronological age (continuous), systolic blood pressure (defined for this analysis as closest blood pressure within 6 months of diagnosis date and two- and five-year post diagnosis), antihypertensive medication use (yes/no), body mass index (continuous), history of smoking within the last year (yes/no), and history of diabetes (yes/no). The upper limit of heart age was set at 100. Excess heart age is equal to the difference between heart age and chronological age and represents the excess risk for cardiovascular events [18].
Second, we examined prevalent elevated excess heart age (an indicator of poorer cardiovascular disease health) defined as an excess heart age ≥ two years at both follow-up timepoints. Third, to examine factors that were associated with a worsening excess heart age, we examined change in excess heart age from baseline (time closest to breast cancer diagnosis and prior to treatment receipt) to follow-up [excess heart age at follow-up – excess heart age at baseline]. Worsening excess heart age was defined as an increase of at least two heart age years, and patients could have “healthy cardiovascular disease risk” (excess heart age less than 0) and become “less healthy” if the change was at least 2 excess heart age years. Among the analytic population of five-year breast cancer survivors, 24 women did not have survey data at two-year follow-up.
Cancer treatments
We examined the following cancer treatments: anthracyclines (yes, no), trastuzumab (yes, no), radiation (yes, no), and endocrine therapy (yes, no, missing). We further assessed radiation by laterality (left, right) due to the potential increased risk of cardiovascular disease after left-sided radiation [5, 19]. Systemic endocrine therapy (including tamoxifen and aromatase inhibitors; yes, no, missing) was determined by use at the 18 months post diagnosis.
Statistical analyses
Descriptive statistics (mean and standard deviation, frequency and percentages) were used to characterize the study population. Paired-sample t-tests and Mcnemar’s chi-square statistics were used to assess differences in excess heart age between baseline and follow-up at two- and five-years.
To determine whether there was an association between cancer treatment and excess heart age (continuous) at two-year follow-up and five-year follow-up, we conducted multivariable linear regression. Further, to assess the association between cancer treatment and 1) prevalent elevated excess heart age (dichotomized) at two-year and five-year follow-up and 2) worsening excess heart age (dichotomized) between baseline and two-year follow-up, between baseline and five-year follow-up, and between two-year and five-year follow-up, we conducted multivariable logistic regression. All models adjusted for age at diagnosis (continuous), race (White versus all other races due to small sample sizes), stage (0, I, II, III), and other cancer treatments (anthracycline, trastuzumab, any radiation, radiation and laterality, or endocrine therapy). All tests were two-sided, and significance was set at p < 0.05. STATA version 17.0 was used.
Results
Study population characteristics
Among 1,302 YWS participants, 372 met inclusion criteria and had survived at least five years after breast cancer diagnosis (Fig. 1). The median age at breast cancer diagnosis was 37.5 years (interquartile range: 35.4–39.6 years) (Table 1). Participants were 93% White, 97% non-Hispanic/Latina, 85% college graduates, 80% married or with a partner, and 68% employed at the time of breast cancer diagnosis. Most patients had either Stage I (40%) or Stage II (39%), and Hormone Receptor [HR] + /HER2-(47%) or HR + /HER2 + breast (21%) cancers. Most (73%) women received chemotherapy, including 40% who received chemotherapy regimens with anthracyclines and no trastuzumab, 8% who received trastuzumab and no anthracyclines, 20% who received both anthracyclines and trastuzumab, and 4% who received other chemotherapy. Nearly 60% of women received radiation, and all women received surgery (30% had breast conserving surgery, 25% unilateral mastectomy, and 45% bilateral mastectomy). None of the 372 women were diagnosed with clinically evident cardiovascular disease after five years of follow-up.
Excess heart age
At baseline, women who went on to receive anthracyclines had slightly higher excess heart age than those who did not (0.40 vs 0.19 years, respectively). The same was observed among those who received radiation versus not (0.88 vs -0.52 years, respectively) (Table 2). Women who went on to receive trastuzumab had a lower excess heart age than those who did not (-0.11 vs 0.49 years, respectively). Over time, the difference in excess heart age between women who received anthracyclines and those who did not increased slightly at two- and five-year follow-ups, whereas the difference between women who received radiation versus those who did not was consistent over time (Fig. 1). Women who received trastuzumab or endocrine therapy had similar excess heart age at follow-up, compared to those who did not receive trastuzumab or endocrine therapy. At two-year follow-up, none of the cancer treatments were significantly associated with increasing excess heart age in the multivariable models (Table 2). However, at five-year follow-up, radiation treatment was associated with increasing excess heart age (β = 1.92, SE = 0.82, p = 0.02), which was largely driven by left-sided radiation (β = 2.49, SE = 0.96, p = 0.01). At five-year follow-up, no associations were observed for anthracyclines, trastuzumab, or endocrine therapy.
Prevalent elevated excess heart age
At two years, 26% (n = 90/348) of young breast cancer survivors had prevalent excess heart age of ≥ 2 years (Table 3). There were no significant associations between the various cancer treatments and prevalent excess heart age at two-years, except for right-sided radiation which had a two-fold increased risk (OR = 2.17, 95%CI = 1.12–4.19). At five years, 27% (n = 100/372) of young breast cancer survivors had a prevalent excess heart age of ≥ 2 years. Any radiation was associated with an increased odds of prevalent elevated excess heart age (OR = 1.92, 95%CI = 1.09–3.41), with left-sided radiotherapy associated with higher odds (OR = 2.13, 95%CI = 1.09–3.41) but the association was attenuated for right-sided radiotherapy (OR = 1.78, 95%CI = 0.94–3.33).
Worsening excess heart age (Increase of ≥ 2 excess heart age years)
Between baseline and two-year follow-up, 22% (n = 75/348) of women had an increase of at least 2 excess heart age years. Between baseline and five-year follow-up, 31% (n = 114/372) women had an increase of at least 2 excess heart age years. Between two-year follow-up and five-year follow-up, 40% (n = 139/372) of women had an increase of at least 2 excess heart age years. Across all three time points, there were no associations between cancer treatments and worsening excess heart age (Supplemental Table 1).
Discussion
In this cohort of young breast cancer survivors, most women had minimal excess heart age at the time of breast cancer diagnosis, suggesting a cardiovascular disease risk comparable to the nationally reported average of excess heart age for women aged 30–39 years [20]. However, nearly 1/3 of young breast cancer survivors in this cohort experienced a change in their excess heart age from breast cancer diagnosis of ≥ 2 years after 5 years of follow-up. Among cancer treatments, radiation, especially left-sided radiation, was associated with higher cardiovascular disease risk after five-years of follow up, while other cancer treatments were not.
While studies have evaluated excess heart age in other patient populations[21,22,23,24,25], only one study has previously assessed excess heart age among breast cancer survivors. This prior study was conducted within an Alabama health system, and the mean excess heart age was 4.2 years at baseline for 152 women under 45 years [15]. The YWS cohort includes mostly non-Hispanic White patients largely based in Massachusetts, who have a statewide reported average excess heart age much lower than Alabama (3.5 vs 8.1 years, respectively) [20]. These differences are likely attributable to regional differences in cardiovascular disease risk factors including body mass index, comorbidities, and physical activity.
We have extended prior research by assessing the association between breast cancer treatment and excess heart age among young five-year survivors in a relatively large prospective cohort (twice the sample size of the Alabama study). We observed higher cardiovascular disease risk at five years for women treated with radiotherapy after breast cancer diagnosis. Cardiovascular disease, especially ischemic heart disease, is a long-term effect of radiation therapy with disease incidence occurring 10 + years after initial treatment [5, 26], with prior studies demonstrating increased risk after left- compared to right-sided radiotherapy [5, 19]. Since none of the patients in our study experienced cardiovascular events at 5 years follow-up, excess heart age may be capturing changes in cardiovascular risk factors, such as increasing blood pressure or body mass index, that may place young female survivors at risk for developing premature radiation-related cardiovascular disease, especially given the accentuated risk due to younger age at radiation exposure [13, 19, 27]. Further, the increased prevalent excess heart age observed after right-sided radiotherapy in one of our models may be related to an indirect effect with obesity, as women who are more obese and have large breasts may be less likely to receive mastectomies due to cosmetic challenges with surgical reconstruction and may be more likely to have partial mastectomy with radiation [28]. These higher-risk women may also have other co-occurring diseases or pre-existing risk factors that could potentially increase their risk of developing cardiovascular disease. Importantly, modern radiation treatment practices for cardioprotection, including positioning to protect the heart such as breath-hold or prone positioning, increased precision using image guidance, and proton therapy as an alternative energy source, could potentially improve long-term radiation-related cardiovascular disease risk [29]; however, we did not have these data available.
In the present analysis, we did not observe associations between excess heart age and anthracyclines, trastuzumab, or endocrine therapy that were found in a prior study [15]. This was reassuring and may be related to increased awareness about potential adverse effects of chemotherapy and resultant healthy lifestyle changes through cancer treatment and survivorship that mitigate cardiovascular disease risk [30]. It is also possible that compared to older patients, young women treated with anthracyclines and trastuzumab may be more resilient against cardiotoxic damage due to the relative lack of baseline cardiovascular disease risk factors [3, 4, 31]. However, it is important to note that subclinical changes in cardiac function that may occur after anthracyclines or trastuzumab receipt, especially when used together, could not be assessed with this excess heart age tool, and risk of cardiovascular disease among young breast cancer survivors may be heightened long after the five years of follow-up in this study as seen in AYA cancer survivors 10 + years after diagnosis [10].
Although we did not observe significant associations between anthracyclines or trastuzumab and excess heart age, clinical guidelines have stressed the importance of monitoring for and preventing cardiomyopathy in patients treated with anthracyclines or trastuzumab since early detection can lead to improved outcomes. Current clinical guidelines for breast cancer survivors do not emphasize screening after radiotherapy [12, 13]; however, we observed significant associations between radiation and subsequent elevated excess heart age. Thus, the clinical implications of this study include cardiovascular surveillance after radiotherapy and management of modifiable lifestyle factors (e.g., physical activity, weight management, hypertension control) to improve cardiovascular disease risk after radiation treatment. Excess heart age could be used in survivorship care to improve provider-patient communication regarding cardiovascular disease risk [18] and could facilitate healthy lifestyle changes or referral for additional screening as well as early detection and intervention for subclinical cardiovascular disease in high-risk women.
Findings of this research should be interpreted in the context of certain limitations, including our inability to assess cardiovascular risk associated with specific chemotherapy regimens (i.e., joint effects of anthracyclines with trastuzumab), radiation dose, or endocrine therapy type (tamoxifen versus aromatase inhibitors) due to inadequate statistical power. Further, five years of follow-up may not be a sufficient length of follow-up for young breast cancer survivors who are relatively healthy at diagnosis and due to the long latency of treatment-related cardiovascular disease, warranting future extended assessments of cardiovascular disease risk (e.g., 10 or more years after breast cancer diagnosis). The tool excess heart age may not fully capture the full range of cardiovascular risk prior to cancer treatment receipt, and we were also unable to assess left ventricular function which may have characterized subclinical changes from diagnosis to after treatment. Finally, the use of heart age is not validated in cancer populations, and studies are needed to inform cardiovascular disease risk associated with potentially cardiotoxic treatments, especially in patients where traditional cardiovascular risk estimation tools are unavailable. Future research should consider the cardiotoxic impact of newer regimens such as immunotherapies and cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors and the potential effects of ovarian suppression on cardiovascular disease risk.
Nevertheless, our finding of potentially increased risk of cardiovascular disease for a substantially small group of young breast cancer survivors, especially after left-sided radiotherapy, warrants future investigation especially given the long survivorship and long latency of cardiovascular disease. Cardiovascular disease risk tools that incorporate cancer treatment predictors into models are needed to appropriately identify high-risk patients, especially among young survivors who have a low absolute, but higher relative risk than age-matched non-cancer controls. Extended follow-up of the YWS cohort as well as evaluation of this risk in other cohorts may further quantify cardiovascular disease risk and long-term cardiac outcomes in young breast cancer survivors.
Data availability
The data that support the findings of this study are from the Young Women’s Breast Cancer Study, but restrictions apply to the availability of these data and are not publicly available.
References
American Cancer Society. Breast Cancer Facts & Figures 2019–2020. Atlanta: American Cancer Society Inc; 2019.
Paluch-Shimon S, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203–17. https://doi.org/10.1016/j.breast.2017.07.017.
Bowles EJA, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. JNCI J Natl Cancer Inst. 2012;104(17):1293–305. https://doi.org/10.1093/jnci/djs317.
Thavendiranathan P, et al. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34(19):2239–46. https://doi.org/10.1200/JCO.2015.65.1505.
Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. https://doi.org/10.1056/NEJMoa1209825.
Khosrow-Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: A population-based cohort study. Circulation. 2020;141(7):549–59. https://doi.org/10.1161/CIRCULATIONAHA.119.044750.
Vo JB et al. Long-term cardiovascular disease risk after anthracycline and trastuzumab treatments in U.S. breast cancer survivors. JNCI J Natl Cancer Inst. 2024. https://doi.org/10.1093/jnci/djae107.
Bhatia S. Long-term complications of therapeutic exposures in childhood: Lessons learned from childhood cancer survivors. Pediatrics. 2012;130(6):1141–3. https://doi.org/10.1542/peds.2012-2884.
Oeffinger KC, Kawashima T, Friedman DL, Kadan-Lottick NS, Robison LL. “Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82.
Chao C, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: The kaiser permanente AYA cancer survivors study. J Clin Oncol. 2016;34(14):1626–33. https://doi.org/10.1200/JCO.2015.65.5845.
Reulen RC, et al. Long-term cause-specific mortality among survivors of childhood cancer. Jama. 2010;304(2):172–9.
Armenian SH, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911. https://doi.org/10.1200/JCO.2016.70.5400.
Mehta LS, et al. Cardiovascular disease and breast cancer: where these entities intersect: A scientific statement from the American heart association. Circulation. 2018;137:30–66. https://doi.org/10.1161/CIR.0000000000000556.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines: Survivorship v2.20). 2020. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf.
Vo JB, Kenzik KM, Landier W, Raju D, Kirklin JK, Meneses K. Excess heart age in young breast cancer survivors over 2-year follow-up. Cancer Causes Control. 2021. https://doi.org/10.1007/s10552-021-01415-3.
Haque R, et al. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2(12):1590–7. https://doi.org/10.1001/jamaoncol.2016.0429.
Ramin C, et al. All-Cause and cardiovascular disease mortality among breast cancer survivors in CLUE II, a long-standing community-based cohort. JNCI J Natl Cancer Inst. 2021;113(2):137–45. https://doi.org/10.1093/jnci/djaa096.
D’Agostino RB, et al. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117(6):743–53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
Carlson LE, et al. Coronary artery disease in young women after radiation therapy for breast cancer. JACC CardioOncology. 2021;3(3):381–92. https://doi.org/10.1016/j.jaccao.2021.07.008.
Yang Q, et al. Vital signs: Predicted heart age and racial disparities in heart age among U.S. adults at the state level. MMWR Morb Mortal Wkly Rep. 2015;64(34):950–8. https://doi.org/10.15585/mmwr.mm6434a6.
Mpofu JJ, et al. Disparities in the prevalence of excess heart age among women with a recent live birth. J Womens Health. 2020;29(5):703–12. https://doi.org/10.1089/jwh.2018.7564.
Hirsch JR, Waits G, Li Y, Soliman EZ. Racial differences in heart age and impact on mortality. J Natl Med Assoc. 2018;110(2):169–75. https://doi.org/10.1016/j.jnma.2017.08.003.
Thompson-Paul AM, et al. Excess heart age in adult outpatients in routine HIV care. AIDS. 2019;33(12):1935–42. https://doi.org/10.1097/QAD.0000000000002304.
Guzman-Vilca WC, Quispe-Villegas GA, Carrillo-Larco RM. Predicted heart age profile across 41 countries: A cross-sectional study of nationally representative surveys in six world regions. eClinicalMedicine. 2022;52:101688. https://doi.org/10.1016/j.eclinm.2022.101688.
Yang Q, Zhang Z, Steele EM, Moore LV, Jackson SL. Ultra-processed foods and excess heart age among U.S. adults. Am J Prev Med. 2020;59(5):e197–206. https://doi.org/10.1016/j.amepre.2020.06.013.
Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–65. https://doi.org/10.1016/S1470-2045(05)70251-5.
Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: Review of an underrecognized pathology. J Am Heart Assoc. 2021;10(18):e021686. https://doi.org/10.1161/JAHA.121.021686.
Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41. https://doi.org/10.1007/s11912-019-0787-1.
Lenihan DJ, Cuculich P. Cardioprotection during therapeutic radiation treatment: peeling the onion of radiation cardiotoxicity? Circ Heart Fail. 2018;11(8). https://doi.org/10.1161/CIRCHEARTFAILURE.118.005294.
Cao Z, Xu C, Yang H, Li S, Wang Y. The role of healthy lifestyle in cancer incidence and temporal transitions to cardiometabolic disease. JACC CardioOncology. 2021;3(5):663–74. https://doi.org/10.1016/j.jaccao.2021.09.016.
Greenlee H, et al. Risk of cardiovascular disease in women with and without breast cancer: The pathways heart study. J Clin Oncol. 2022;40:1647–58. https://doi.org/10.1200/JCO.21.01736.
Funding
Open access funding provided by the National Institutes of Health. The Young Women’s Breast Cancer Study is funded in part by Susan G. Komen (AHP) and Breast Cancer Research Foundation (AHP). JBV was supported by the Cancer Prevention Fellowship Program at the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
J.B.V. and A.H.P. wrote the main manuscript text. J.B.V. conducted all analyses and prepared corresponding tables and figures. C.S., G.K., B.X.Z., S.R., R.G., and A.H.P. provided acquisition of data and administrative, technical, and material support. All authors edited and reviewed the manuscript drafts and provided input on the analyses, tables, and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vo, J.B., Rosenberg, S., Zhang, B.X. et al. Association of cancer treatment with excess heart age among five-year young breast cancer survivors. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-024-01645-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-024-01645-9