Abstract
Purpose
The primary goal of this scoping review was to summarize the literature published after the 2018 National Cancer Institute think tank, “Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors,” on physical and cognitive functional outcomes among cancer survivors treated with chemotherapy. We focused on the influence of chemotherapy on aging-related outcomes (i.e., physical functional outcomes, cognitive functional outcomes, and frailty), given the known associations between chemotherapy and biologic mechanisms that affect aging-related physiologic processes.
Methods
A search was conducted across electronic databases, including PubMed, Scopus, and Web of Science, for manuscripts published between August 2018 and July 2023. Eligible studies: 1) included physical function, cognitive function, and/or frailty as outcomes; 2) included cancer survivors (as either the whole sample or a subgroup); 3) reported on physical or cognitive functional outcomes and/or frailty related to chemotherapy treatment (as either the whole sample or a subgroup); and 4) were observational in study design.
Results
The search yielded 989 potentially relevant articles, of which 65 met the eligibility criteria. Of the 65 studies, 49 were longitudinal, and 16 were cross-sectional; 30 studies (46%) focused on breast cancer, 20 studies (31%) focused on the age group 60 + years, and 17 (26%) focused on childhood cancer survivors. With regards to outcomes, 82% of 23 studies reporting on physical function showed reduced physical function, 74% of 39 studies reporting on cognitive functional outcomes found reduced cognitive function, and 80% of 15 studies reporting on frailty found increasing frailty among cancer survivors treated with chemotherapy over time and/or compared to individuals not treated with chemotherapy. Fourteen studies (22%) evaluated biologic mechanisms and their relationship to aging-related outcomes. Inflammation was consistently associated with worsening physical and cognitive functional outcomes and epigenetic age increases. Further, DNA damage was consistently associated with worse aging-related outcomes.
Conclusion
Chemotherapy is associated with reduced physical function, reduced cognitive function, and an increase in frailty in cancer survivors; these associations were demonstrated in longitudinal and cross-sectional studies. Inflammation and epigenetic age acceleration are associated with worse physical and cognitive function; prospective observational studies with multiple time points are needed to confirm these findings.
Implications for cancer survivors
This scoping review highlights the need for interventions to prevent declines in physical and cognitive function in cancer survivors who have received chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
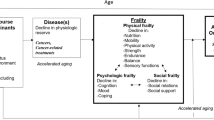
The growing population of cancer survivors is a result of advances in cancer screening, diagnosis, treatment, and supportive care [1, 2]. As of January 1, 2022, it was estimated that there were over 18 million Americans with a history of cancer [3]. With improvements in cancer care extending survival, aging-related physical and cognitive functional changes, frailty, and quality of life become even more important to understand and evaluate in cancer survivors [4, 5]. The concept of accelerated aging refers to the process whereby an individual experiences aging-related changes at a faster rate than average [6]. Aging-related functional declines as a consequence of cancer and its treatment are associated with several biologic mechanisms, including DNA damage, epigenetic dysregulation, mitochondrial damage, cellular senescence, oxidative stress, and chronic inflammation [7]. Cancer survivors encounter functional declines typically associated with aging at earlier chronological ages than their cancer-free counterparts [8, 9].
The National Cancer Institute (NCI) organized a think tank in 2018 [10] to review and summarize the state of the science related to measuring and identifying aging phenotypes in cancer survivors. Participants at the “Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors” think tank reviewed a framework proposed by Ahles and Hurria, positing that cancer and/or cancer treatment could lead to an aging trajectory that is shifted (i.e., Accentuated Aging Hypothesis) or an aging trajectory with an increased rate of functional decline (i.e., Accelerated Aging Hypothesis) [11]. Based on available evidence, the 2018 think tank participants hypothesized that chemotherapy may substantially affect aging-related physical and functional outcomes through increased inflammation, persistent DNA damage, decreased telomere length, and other mechanisms [10]. The think tank summary concluded, "More research is needed to better assess the rate of aging and to understand the relationships between markers of biological age and functional outcomes in cancer survivors” [10].
In this scoping review, we provide an update on the scientific evidence generated since the 2018 NCI think tank [10]. We focus on aging-related outcomes (i.e., physical function, cognitive function, and frailty) in cancer survivors after chemotherapy, given the published evidence demonstrating associations between chemotherapy and aging-related biologic mechanisms [10]. We summarize observational studies (i.e., cross-sectional and longitudinal) published after the 2018 NCI think tank that investigate the relationships between chemotherapy and physical functional outcomes, cognitive functional outcomes, and frailty among cancer survivors. We report on the relationships between biologic mechanisms and aging-related outcomes in these studies.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) recommendations (Data Supplement) [12]. Essential components of a PRISMA scoping review include a systematic search strategy, clear inclusion criteria, presentation of the study selection processes, and synthesis of key findings of the included studies [12]. We collaborated with a health sciences librarian (JM) to design a comprehensive search of relevant databases to identify literature evaluating physical functional outcomes, cognitive functional outcomes, and frailty among cancer survivors treated with chemotherapy. We searched for articles from the following databases published between August 2018 and July 2023: PubMed, Web of Science, and Embase. The search involved the integration of standardized terms to retrieve studies about cancer survivors, accelerated aging, functional changes, cognitive changes, and frailty. The specific search terms are detailed in the Data Supplement. We also identified additional relevant studies by screening the reference lists of relevant articles (i.e., “snowball” search). The snowball strategy is approved per PRISMA guidelines [13,14,15,16].
Inclusion criteria
Studies were included if they investigated aging-related outcomes (i.e., physical functional outcomes, cognitive functional outcomes, and/or frailty) among cancer survivors treated with chemotherapy. To extend the findings from the 2018 think tank [10], studies needed to (a) evaluate physical function, cognitive function, and/or frailty as outcomes; (b) include patients with cancer (either the whole sample or a subgroup); (c) include individuals who received chemotherapy treatment (either the whole sample or a subgroup); (d) be observational studies (i.e., employ cross-sectional or longitudinal design); and (e) be written in English. Physical functional outcomes included one or more of these categories: 1) physical performance (e.g., objective tests of gait speed, lower extremity performance, physical activity); 2) patient-reported functional status (e.g., self-reported ability to complete daily tasks such as bathing or cooking), 3) and health-related quality of life (HRQOL) with physical functional components. We included studies that evaluated cognitive functional outcomes and frailty with self-reported and/or objective measures outlined in the 2018 think tank [10, 17, 18]. In some cases, manuscripts were identified by our search that included both the aging-related outcomes of interest and biologic measures (e.g., epigenetic markers, inflammatory markers). In addition to synthesizing information on the relationship between chemotherapy and aging-related outcomes, we summarize the relationships between biologic measures and these outcomes.
Exclusion criteria
The exclusion criteria for this review were as follows: (a) abstract only; (b) languages other than English; (c) review article, interventional trial, and case study; (d) did not include patients with cancer who received chemotherapy; (e) studies that examined biologic measures without linkage to patient-oriented aging-related outcomes; (f) studies that examined the effects of hormonal therapy, radiation, or surgery alone.
Search strategy/data charting
Two researchers (MM and MA) reviewed the articles independently to check for the inclusion/exclusion criteria by title and abstracts. After downloading or ordering each report’s full text, the two reviewers thoroughly examined eligibility again and, if included, extracted the data (described below). Duplicate articles were excluded. Disagreements between the two reviewers at each step were resolved by consensus after reviewing the full text, or if consensus could not be reached, a third researcher (SM) made the final decision.
The following information was extracted from each article: first author, year of publication, country of the first author, type of study, sample size at baseline, type of cancer, chemotherapy history, age group, assessment time points, description of how aging-related outcome was measured, and primary findings. Whenever possible, we assessed changes in outcomes over time, accelerated aging (if three or more time points and a longitudinal comparison group with similar time-point assessment intervals were included), and if the investigators reported that the results were statistically significant and/or clinically meaningful. The reported sample sizes exclude the number of healthy participants who were not diagnosed with cancer.
Results
Identification of relevant studies (Fig. 1)
Our search yielded 989 potentially relevant articles (968 articles through the databases searched and 21 articles through the snowball search). After removing duplicates and articles published before the specified timeframe, 474 unique articles remained (Fig. 1). These articles were screened for eligibility, and 371 were determined not to meet the inclusion criteria. The remaining 103 articles underwent full-text assessment, leading to the exclusion of an additional 38 articles. Ultimately, 65 articles that met the inclusion criteria were included. Figure 1 depicts the study selection process.
Study characteristics (Table 1)
Studies were published between August 2018 and July 2023. Most studies were conducted in the United States (n = 50). With respect to publication type, 49 articles were longitudinal studies (Tables 2, 3 and 4), and 16 were cross-sectional studies (Supplemental Table 1). The cancer survivors included in these studies ranged from 18 to 88 years of age. Twenty studies (31%) were limited to the age group 60 + [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], while 17 studies (26%) were limited to childhood cancer survivors [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The time since cancer diagnosis varied widely, ranging from one month to 30 years. The most common types of cancer were breast cancer (46%) [19, 22,23,24, 26,27,28,29,30,31, 33,34,35, 38, 57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], followed by lymphomas, leukemias, sarcomas (20%) [32, 39,40,41,42, 44, 45, 47, 48, 52, 55, 73, 74], gastrointestinal cancers (8%) [37, 54, 75,76,77,78] and lung cancer (3%) [79, 80]. Study characteristics, including the age group of patients enrolled and their cancer types, outcomes examined, and number of time points, are summarized in Table 1.
Aging-related outcomes
Cognition was the most frequently investigated outcome, with 60% of the studies assessing cognitive functional outcomes. Physical functional outcomes were examined in 35% of the studies. Frailty was investigated in 23% of the studies. Eighteen percent of the studies examined more than one outcome (e.g., cognition and frailty).
Studies examining cognitive functional outcomes among cancer survivors (n = 39)
Thirty-nine studies (60%) examined cognitive functional outcomes among cancer survivors; 32 were longitudinal in design (Table 2). Twenty-one studies (n = 21/39; 54%) examined cognitive functional outcomes over three or more time points [22, 28, 30, 31, 36, 42, 47,48,49, 59, 60, 66, 68,69,70, 73, 75, 77, 78, 81]; of these, 19 (n = 19/21, 90%) included a comparison group, allowing for an assessment of possible accelerated aging [28, 31, 33, 36, 42, 47,48,49, 59, 66, 68,69,70, 73, 75, 77, 78, 81]. The most commonly used cognitive measures were Trail Making Test A/B (n = 14/39; 36%) [22,23,24, 28, 30, 48, 49, 52, 53, 59, 66, 71, 73, 84] and Mini-Mental State Examination (MMSE) (n = 4/39; 10%) [32, 75, 78, 81]. Examples of other measures used to assess cognition include the Cambridge Neuropsychological Test Automated Battery (CANTAB) [59, 73], NIH Toolbox for the Assessment of Neurological and Behavior Function Cognition (NIH-TB) [53], Montreal Cognitive Assessment (MoCA) [77], and Controlled Word Association Test [41, 53, 59]. For self-report measures, the Functional Assessment of Cancer Therapy-Cognition (FACT-Cog) was commonly used (n = 9/39; 23%) [22, 31, 61, 63, 69, 72, 73, 78, 82] followed by Childhood Cancer Survivor Study Neurocognitive Questionnaire (CCSS NCQ; n = 4/39; 10%) [43,44,45, 47]. Among 19 longitudinal studies examining patients with a parallel comparison group, each with three or more time points, 16 (n = 16/19; 84%) found that cancer survivors receiving chemotherapy developed cognitive decline based on one or more of tests, relative to a control group assessed at similar time intervals, indicating possible accelerated cognitive aging [22, 28, 31, 33, 36, 42, 47,48,49, 59, 66, 68,69,70, 73, 75, 77, 78, 81]. Six of these studies (n = 6/16; 37%) reported clinically meaningful differences between survivors and controls [22, 31, 42, 49, 70, 73]. Only three studies (n = 3/19; 16%) found no difference in cognitive functional outcomes pre- to post-chemotherapy or between those who received chemotherapy and the healthy control groups [37, 78, 81]. In one of these studies, the majority of patients with cancer did not receive chemotherapy; when the patients were stratified based on the types of cancer treatment they received, those who received chemotherapy declined slightly faster than other groups [81].
The majority of research on cancer-related cognitive function has focused on adults with breast cancer. In 580 patients with breast cancer aged 21 + years, Janelsins et al. found evidence of chemotherapy-related cognitive impairment (CRCI) in multiple domains for at least six months post-chemotherapy, with a difference noted for visual memory compared to controls [59]. Using a variety of patient-reported and objective measures, several studies have demonstrated cognitive functional declines in older patients with breast cancer receiving chemotherapy [22, 31, 70]. For example, Mandelblatt et al. reported impaired attention and reduced processing speed and executive function for up to two years after chemotherapy in 344 patients aged 60 + with breast cancer compared to non-cancer controls [22]; these data are suggestive of accelerated cognitive aging.
Multiple studies have investigated cognitive functional outcomes among childhood cancer survivors. In a study of pediatric cancer survivors aged 6–17 years old by Chipeeva et al., survivors (n = 504) scored significantly worse on measures of memory, visuospatial processing, and verbal fluency than those without a history of cancer [41]. In two studies by Olsson et al., including childhood cancer survivors diagnosed with Wilms tumor and soft tissue sarcoma, survivors had lower scores than community controls in verbal reasoning, word reading, mathematics, sustained attention, long-term verbal memory, and verbal fluency [48, 49]. In a cross-sectional analysis of survivors of Hodgkin’s lymphoma, Williams et al. demonstrated that survivors, compared with their siblings, exhibited impairment in neurocognitive function and were more likely to be unemployed and have a lower income [45]. In a large study by Phillips et al. of childhood cancer survivors (n = 2375) assessed up to 35 years post-cancer diagnosis, new-onset memory impairment emerged more often in cancer survivors than in their siblings [85]. The increased risk was associated with cancer treatment, modifiable health behaviors, and chronic health conditions. Similarly, in a cohort study including 960 childhood cancer survivors aged < 21 years at diagnosis, Kedan-Lottick et al. showed that survivors of childhood osteosarcoma and Ewing sarcoma were at increased risk for reporting neurocognitive difficulties, which were associated with employment status and chronic health conditions that developed over time [44].
Studies examining physical functional outcomes among cancer survivors (n = 23)
Twenty-three studies (35%) examined physical functional outcomes among cancer survivors; 17 were longitudinal in design (Table 3). Sixteen studies (n = 16/23; 69%) examined physical functional outcomes over three or more time points [19,20,21, 26, 34, 35, 47,48,49, 57, 58, 69, 78,79,80, 83]; eight (n = 8/23; 35%) included a comparison group allowing for an assessment of possible accelerated aging in this domain [34, 47,48,49, 58, 69, 78, 83]. Across studies, a broad spectrum of measures evaluating various physical functional outcomes were used. Most studies (n = 20/23; 87%) used patient-reported outcomes (PRO) to assess physical function among cancer survivors. The PRO most commonly utilized was the Medical Outcomes Study Questionnaire Short Form -36 (SF-36) (n = 12/23 studies; 52%) [29, 40, 45, 47,48,49, 56,57,58, 79, 83]. Among three studies (n = 3/23; 13%)[29, 52, 69] that examined objective physical performance only, the most common measure was the Short Physical Performance Battery (SPPB) test. Overall, 19/23 (82%) of studies documented an association of chemotherapy with reduced physical function over time in longitudinal studies and/or differences in physical functional outcomes between those participants who received chemotherapy and those who did not [19,20,21, 26, 34, 35, 40, 45, 48, 57, 58, 79, 80, 83]. The six cross-sectional studies demonstrated physical function differences among cancer survivors receiving chemotherapy compared to participants who did not [32, 38, 40, 45, 56, 86]. Among the 16 longitudinal studies with at least three assessment points, 81% (n = 13/16) demonstrated statistically significant changes over time [19,20,21, 26, 34, 38, 47, 48, 57, 58, 69, 80, 83]. Among the eight longitudinal studies with a comparison group, 75% (n = 6/8) showed greater physical functional declines over time in cancer survivors than in controls, indicating evidence of accelerated aging among cancer survivors [34, 47, 48, 58, 69, 83].
Twelve studies (n = 12/23, 52%) examined long-term changes (≥ 5 years) since diagnosis [26, 34, 40, 45, 47,48,49, 52, 57, 58, 78, 83]. Five studies examined physical functional outcomes for up to one year [19,20,21, 79, 80]. As examples, Medysky et al. and Presley et al. found that patients with lung cancer aged 18 + years old who had a high level of symptom burden were more likely than those who had a lower symptom burden to experience physical functional declines as measured by HRQOL measures over one year after diagnosis [21, 79].
The majority of longitudinal studies (n = 12/16; 75% of studies that include three or more time points) provided data suggestive of accelerated aging among cancer survivors compared to non-cancer controls. For example, Stefanski et al. found that acute myeloid leukemia survivors treated with intensive chemotherapy were more likely than their siblings to report impairment in physical function as measured by SF-36 scores [47]. In another study of 9203 patients with various cancer types, Cespedes-Feliciano et al. found that cancer survivors aged 50 + years old experienced accelerated declines in physical function post-diagnosis compared to controls [83].
Studies conducted in older patients found physical function declines were common after chemotherapy [19, 29, 34, 57, 58]. Hurria et al. found that of 42% of 256 patients with breast cancer who experienced physical function declines on an HRQOL measure at the end of chemotherapy, only 47% recovered by 12 months [19]. Winters-Stone et al., Avis et al., Micheal et al., and Rentscher et al. found that older survivors of breast cancer were more likely to experience physical functional declines than those without breast cancer [29, 34, 57, 58].
Studies examining frailty outcomes among cancer survivors (n = 15)
Among 15 studies (n = 15/65; 23%) that assessed frailty in patients receiving chemotherapy, eight (n = 8/15; 53%) were longitudinal in design (Table 4) [25, 28, 42, 50, 54, 61, 65, 67]. Only six studies (n = 6/15; 40%) examined frailty over three or more time points [28, 42, 54, 61, 65, 67]; five (n = 5/6; 83%) of these studies included a comparison group [28, 42, 54, 61, 65, 67]. Ten studies (n = 10/15; 67%) used the Fried frailty phenotype index (in its original or modified version) [39, 40, 42, 50, 51, 55, 56, 61, 65, 67], while five studies (n = 5/15; 33%) utilized the deficit accumulation index (DAI) [24, 27, 28, 54, 62]. Seven studies (n = 7/15; 47%) examined frailty in a cohort of childhood cancer survivors [39, 40, 42, 50, 51, 54,55,56], while four studies (n = 4/15; 27%) examined frailty among older adults [24, 27, 28, 62]. All five longitudinal studies that included a comparison group found that cancer survivors experienced increased frailty compared to non-cancer controls [28, 42, 54, 61, 65, 67]. Williams et al. found that childhood cancer survivors aged 18 + years (n = 400) had increased frailty as measured by DAI compared with controls [54]. Another study by Magnuson et al. found that among breast cancer survivors aged 50 + years old, longitudinal declines in FACT-Cog and objective measures of attention and memory were associated with increased frailty during chemotherapy and for up to six months post-chemotherapy compared with controls [61].
Studies examining biologic measures in relation to physical functional outcomes, cognitive functional outcomes, or frailty (Supplementary Table 2)
Overall, 14 studies (22%) identified in our search examined the relationship between biologic measures and aging-related outcomes among cancer survivors; four of these studies (n = 4/14; 29%) had an endpoint of frailty [55, 56, 65, 66], two (n = 2/14; 14%) had an endpoint of physical function [34, 56], and nine had an endpoint of cognitive function (9/14, 64%) [30, 31, 33, 60, 63, 64, 66, 68, 71]. Two of these studies (n = 2/14; 14%) were conducted with childhood cancer survivors [55, 56], while four studies (n = 4/14; 29%) were restricted to older cancer survivors [30, 31, 33, 34]. Most (n = 12/14; 86%) of the studies were conducted with breast cancer survivors [30, 31, 33, 34, 60, 63,64,65,66,67,68, 71].
Six studies (n = 6/14; 43%) examined the association of inflammatory markers (e.g., cytokines, immune cells) and aging-related outcomes [31, 65,66,67,68, 71]. In a longitudinal study involving 144 patients with breast cancer aged 50 + , Gilmore et al. reported a correlation between elevated serum levels of interleukin (IL)-6 and soluble tumor necrosis factor-alpha (TNF-alpha) with increased frailty post-chemotherapy [65]. Another study by Gilmore et al. found a positive association between pre-chemotherapy neutrophil to lymphocyte ratio (NLR) and post-chemotherapy frailty in 586 patients with breast cancer [67]. In a longitudinal study with 400 older breast cancer survivors, Carroll et al. found that higher C-reactive protein levels predicted lower self-reported cognition [31]. Belcher et al. found that higher IL-8 levels were associated with worse attention, while higher IL-4 and IL-10 levels were linked to better performance on cognitive measures [66].
Another eight studies (n = 8/14; 57%) examined the association of epigenetic markers, telomere length, and DNA damage with aging-related outcomes [30, 33, 34, 55, 56, 60, 63, 64]. Carroll et al. and Alhareeri et al. found that greater DNA damage and lower telomerase activity were related to worse cognitive function [60, 64]. Yao et al. found an association between epigenetic changes in leukocyte DNA methylome and self-perceived cognitive decline in breast cancer survivors [63]. Gehle et al. and Smitherman et al. found that in 60 childhood cancer survivors, frailty status was associated with a faster pace of epigenetic aging and higher levels of p16INK4a, a marker of cellular senescence [55, 56].
Discussion
This review summarizes the plethora of research published since the 2018 NCI think tank evaluating whether chemotherapy affects cognitive and physical functional outcomes and frailty in cancer survivors [10]. As revealed by this scoping review, a major advance in the field has been the emergence of research findings from several larger longitudinal studies with well-matched comparator groups and relatively high retention rates, allowing for assessing the impact of cancer and chemotherapy over time. As recommended by the think tank, many studies evaluated data from multiple time points along the treatment continuum (i.e., pre-treatment, early treatment phase, shortly after or six months post-chemotherapy to long-term survivorship), allowing for examination of changes over time. Overall, 8/23 (35%), 19/39 (49%), and 5/15 (33%) studies included three or more time points and a comparator group for evaluation of physical function, cognitive function, and frailty changes over time, respectively, providing for assessment of accelerated aging patterns. Cancer survivors with various cancer types were included in the studies identified, although a predominance of studies included breast cancer survivors only. Several studies since the think tank used recommended measures of cognitive function, physical function and frailty, and included recommended usage of objective and self-report measures. Across the studies included in this review, there was consistent evidence of worsening physical function [34, 47, 48, 58, 69, 83], cognitive function [22, 28, 31, 33, 36, 42, 47,48,49, 59, 66, 68,69,70, 73, 75, 77, 78, 81], and indicators of frailty [28, 42, 54, 61, 65, 67] in cancer survivors over time after chemotherapy, with greater declines in cancer survivors over time compared to individuals without cancer in 27/32 (84%) of these studies.
Cognitive function was the most commonly examined outcome among the aging-related outcomes chosen for this review. Several studies used International Cancer and Cognition Task Force recommended assessments (Trail-making Test was most widely used), as well as those recommended for inclusion in geriatric assessment for cognitive screening in older adults (MMSE was most commonly used) and neuroscience-based measures [59, 87, 88]. The cognitive domain was most frequently investigated with objective neurocognitive assessment batteries in studies with multiple time points and a comparator group. Longitudinal and cross-sectional studies showed differences in cognitive functional outcomes between cancer survivors and those without cancer, and the highest quality studies included a comparator group of similar age, sex, and educational level. Importantly, cognitive changes were identified in cancer survivors in various age groups addressing recommendations from the think tank to address the changes across the lifespan. Studies demonstrated cognitive functional declines in middle-aged and older adult populations receiving chemotherapy compared to age-matched controls who did not receive chemotherapy, supporting accelerated aging [22, 28, 31, 33, 42, 47, 59, 66, 68,69,70, 73, 75, 77, 81]. Cross-sectional studies also revealed cognitive functional outcome differences between childhood cancer survivors and age-matched controls [45, 55]. Multiple studies demonstrated that cognitive deficits could persist for even several years after treatment [42, 48, 49, 52]; however, longer-term follow-up data that evaluate survivors 5 to 10 years post-therapy are still needed.
Most studies demonstrated evidence of physical functional declines [19,20,21, 26, 34, 47, 48, 57, 58, 69, 80, 83]. Physical function was evaluated using patient-reported measures recommended by the 2018 think tank [10], such as Instrumental Activities of Daily Living and self-reported difficulties on physical tasks; the majority of studies utilized validated HRQOL scales (e.g., SF-36) [40, 45, 47,48,49, 56,57,58, 79, 83]. Aging-sensitive objective physical performance measures (e.g., Timed Up and Go; gait speed), as recommended by the 2018 think tank, were not frequently utilized to assess physical function. Most longitudinal studies demonstrated physical functional declines, with baseline symptoms, disability, and cognitive function increasing the likelihood of physical functional decline [21, 35]. In studies with several time points that included a comparator group, cancer survivors showed an increased rate of physical functional decline using both patient-reported and objective measures, demonstrating evidence for accelerated aging [34, 47, 48, 58, 69, 83].
The Studies predominantly utilized Fried's frailty criteria and the DAI to measure frailty. Findings consistently pointed to increased frailty among childhood cancer survivors over time and compared to those without cancer, indicating that frailty can be a substantial issue for this population [39, 40, 42, 50, 51, 54,55,56]. While frailty is usually considered an aging-related condition in older adults [28], recent research suggests frailty characteristics develop following chemotherapy in adults in midlife [40]; confirmatory studies are needed to validate these findings. Similarly, childhood cancer survivors accumulate deficits for years following their cancer diagnosis [54]. Future prospective cohort studies should address frailty trajectories over time in middle-aged adults and pediatric and young adult populations.
Biologic processes linked to functional impairments and decline may be useful biomarkers for understanding how biologic processes change over time with respect to functional decline and for predicting the likelihood of worsening in function. In general, inflammation has been consistently shown to be associated with greater frailty and worse cognitive functional outcomes [31, 65,66,67,68, 71] in studies of innate inflammation and specific immune-mediated effector signaling molecules. An avenue of future research in this area is to comprehensively understand how networks of inflammatory processes track over time from pre-treatment, during treatment, and post-treatment in patients with different trajectories of functional decline. Further, it will be important to understand which markers may contrast those with progressive declines with those who improve over time.
Genetics and epigenetics may also help understand the risk of functional decline and accelerated aging. For example, the APOE4 genotype was associated with cognitive decline in patients receiving chemotherapy compared to those receiving hormonal therapy [30]. While these findings need to be validated in larger studies, APOE4, a marker associated with dementia risk, may be a biomarker for cancer-related cognitive decline. Preliminary epigenetic studies have shown that survivors with greater epigenetic aging reported more cognitive impairment than survivors without epigenetic age increases [56, 60, 63, 64]. It will be important to validate these preliminary findings and further understand the functional implications of specific epigenetic signatures closely linked to aging-related phenotypes and accelerated aging in cancer survivors across the lifespan.
Limitations and gaps
Since our scope was to provide a broad overview of recent research that assessed physical functional and cognitive changes and overall frailty among cancer survivors, it is possible that other studies, especially those with terms not included in our eligibility search criteria, may have been missed. We did not include specific biologic mechanisms as search terms; instead, we reported on biologic mechanisms associated with aging-related outcomes in the manuscripts we identified using the employed search terms. A more comprehensive systematic review of biologic contributors of functional changes across the hallmarks of aging and predictors of worsening function is warranted, as we limited our discussion on biology to studies identified from the search. We did not include “attenuated aging” as a search term because these analyses are usually embedded in studies evaluating “accelerated aging,” which we did include as a search term. Further, we did not search using terms for specific subdomains of cognitive function (e.g., memory), physical function (e.g., balance), and frailty (e.g., fatigue). While our review did reveal findings on functional subdomains identified from the search terms used, since we were not explicit on all subdomains, we likely missed studies that focused on the influence of chemotherapy on specific subdomains.
Future research directions
To continue to make progress, longitudinal studies that evaluate aging-related outcomes over extended periods from diagnosis to years post-treatment are needed; these studies should integrate multiple time points (e.g., pre-treatment, during treatment, post-treatment, and at several follow-ups) and collect data on differing trajectories of patients over time (e.g., patients who improve, remain stable, decline) to increase understanding of which survivors are resilient or recover over time and which continue to decline. Investigators should consistently report whether the results are statistically significant or clinically meaningful. Research efforts should include diverse cancer types to enhance the generalizability of findings. As the landscape of cancer treatment continues to evolve, there is an urgent need for research examining the impact of new modalities and therapies on accelerated aging in cancer survivors. Future studies should investigate the role of social determinants of health, including socioeconomic status, access to healthcare, and social support, which can provide valuable insights into the broader determinants influencing the aging process in cancer survivors. The effects of specific chemotherapy regimens on aging-related outcomes are still largely unknown; future research should evaluate these effects in prospective studies, and systematic reviews or meta-analyses can be considered when there is a more robust evidence base. Additionally, future studies should delve into the mechanisms associated with accelerated aging. Exploring the molecular and cellular pathways (inflammation, epigenetic changes) related to physical and cognitive functional declines may help identify at-risk patients, monitor their physical and cognitive function over time, and ultimately guide targeted therapeutic strategies to mitigate these aging-related consequences.
Data availability
All data generated during this study are included in this published article and its supplementary files.
References
Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. “Cancer survivors: a booming population,” (in eng). Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. https://doi.org/10.1158/1055-9965.Epi-11-0729.
Rowland JH, Bellizzi KM. “Cancer survivorship issues: life after treatment and implications for an aging population,” (in eng). J Clin Oncol. 2014;32(24):2662–8. https://doi.org/10.1200/jco.2014.55.8361.
Miller KD, et al. “Cancer treatment and survivorship statistics, 2022,” (in eng). CA Cancer J Clin. 2022;72(5):409–36. https://doi.org/10.3322/caac.21731.
Flannery MA, Culakova E, Canin BE, Peppone L, Ramsdale E, Mohile SG. “Understanding Treatment Tolerability in Older Adults With Cancer,” (in eng). J Clin Oncol. 2021;39(19):2150–63. https://doi.org/10.1200/jco.21.00195.
Nightingale G, et al. “Perspectives on functional status in older adults with cancer: An interprofessional report from the International Society of Geriatric Oncology (SIOG) nursing and allied health interest group and young SIOG,” (in eng). J Geriatr Oncol. 2021;12(4):658–65. https://doi.org/10.1016/j.jgo.2020.10.018.
Margolick JB, Ferrucci L. “Accelerating aging research: how can we measure the rate of biologic aging?,” (in eng). Exp Gerontol. 2015;64:78–80. https://doi.org/10.1016/j.exger.2015.02.009.
Sedrak MS, Kirkland JL, Tchkonia T, Kuchel GA. “Accelerated aging in older cancer survivors,” (in eng). J Am Geriatr Soc. 2021;69(11):3077–80. https://doi.org/10.1111/jgs.17461.
Kadambi S, et al. Older adults with cancer and their caregivers — current landscape and future directions for clinical care. Nat Rev Clin Oncol. 2020;17(12):742–55. https://doi.org/10.1038/s41571-020-0421-z.
Carroll JE, Bower JE, Ganz PA. “Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care,” (in eng). Nat Rev Clin Oncol. 2022;19(3):173–87. https://doi.org/10.1038/s41571-021-00580-3.
Guida JL, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. Jnci-J Nat Cancer Inst. 2019;111(12):1245–54. https://doi.org/10.1093/jnci/djz136. (Art no. djz136).
Ahles TA, Hurria A. “New Challenges in Psycho-Oncology Research IV: Cognition and cancer: Conceptual and methodological issues and future directions,” (in eng). Psychooncology. 2018;27(1):3–9. https://doi.org/10.1002/pon.4564.
Tricco AC, et al. “PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation,” (in eng). Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/m18-0850.
Choong MK, Galgani F, Dunn AG, Tsafnat G. “Automatic evidence retrieval for systematic reviews,” (in eng). J Med Internet Res. 2014;16(10):e223. https://doi.org/10.2196/jmir.3369.
Jacobsen PB, et al. “Systematic Review of the Impact of Cancer Survivorship Care Plans on Health Outcomes and Health Care Delivery,” (in eng). J Clin Oncol. 2018;36(20):2088–100. https://doi.org/10.1200/jco.2018.77.7482.
Mohamed MR, et al. “Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis,” (in eng). Oncologist. 2020;25(1):e94–108. https://doi.org/10.1634/theoncologist.2019-0406.
Creagh NS, et al. “Self-Collection Cervical Screening in the Asia-Pacific Region: A Scoping Review of Implementation Evidence,” (in eng). JCO Glob Oncol. 2023;9: e2200297. https://doi.org/10.1200/go.22.00297.
Guralnik JM, Branch LG, Cummings SR, Curb JD. “Physical performance measures in aging research,” (in eng). J Gerontol. 1989;44(5):M141–6. https://doi.org/10.1093/geronj/44.5.m141.
Reuben DB, Siu AL, Kimpau S. “The predictive validity of self-report and performance-based measures of function and health,” (in eng). J Gerontol. 1992;47(4):M106–10. https://doi.org/10.1093/geronj/47.4.m106.
Hurria A, et al. “Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer,” (in eng). J Am Geriatr Soc. 2019;67(5):920–7. https://doi.org/10.1111/jgs.15493.
Wong ML, et al. “Characteristics Associated With Physical Function Trajectories in Older Adults With Cancer During Chemotherapy,” (in eng). J Pain Symptom Manage. 2018;56(5):678-688.e1. https://doi.org/10.1016/j.jpainsymman.2018.08.006.
Presley CJ, et al. “Functional trajectories before and after a new cancer diagnosis among community-dwelling older adults,” (in eng). J Geriatr Oncol. 2019;10(1):60–7. https://doi.org/10.1016/j.jgo.2018.05.017.
Mandelblatt JS, et al. “Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study,” (in eng). J Clin Oncol. 2018;36(32):Jco1800140. https://doi.org/10.1200/jco.18.00140.
Lange M, et al. “Cognitive Changes After Adjuvant Treatment in Older Adults with Early-Stage Breast Cancer,” (in eng). Oncologist. 2019;24(1):62–8. https://doi.org/10.1634/theoncologist.2017-0570.
Root JC, et al. “Cognitive Aging in Older Breast Cancer Survivors,” (in eng). Cancers (Basel). 2023;15(12):3208. https://doi.org/10.3390/cancers15123208.
Ji J, Sun CL, Cohen HJ, Muss HB, Bae M, Sedrak MS. “Toxicity risk score and clinical decline after adjuvant chemotherapy in older breast cancer survivors,” (in English). J Nat Cancer Inst. 2023;115(5):578–85. https://doi.org/10.1093/jnci/djad029.
Lemij AA, et al. “Physical Function and Physical Activity in Older Breast Cancer Survivors: 5-Year Follow-Up from the Climb Every Mountain Study,” (in English). Oncologist, Article. 2023;28(6):E317–23. https://doi.org/10.1093/oncolo/oyad027.
Ahles TA, et al. (2023) "Cognitive function is mediated by deficit accumulation in older, long-term breast cancer survivors." J Cancer Survivorship. https://doi.org/10.1007/s11764-023-01365-6.
Ahles TA, et al. Relationship between cognitive functioning and frailty in older breast cancer survivors. J Geriatr Oncol. 2022;13(1):27–32. https://doi.org/10.1016/j.jgo.2021.07.011.
Winters-Stone KM, Medysky ME, Savin MA. Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol. 2019;10(2):311–6. https://doi.org/10.1016/j.jgo.2018.10.006.
Van Dyk K, et al. (2021) "Protective Effects of APOE ϵ2 Genotype on Cognition in Older Breast Cancer Survivors: The Thinking and Living with Cancer Study," (in English). JNCI Cancer Spectrum 5(2). https://doi.org/10.1093/jncics/pkab013.
Carroll JE, et al. “Elevated C-Reactive Protein and Subsequent Patient-Reported Cognitive Problems in Older Breast Cancer Survivors: The Thinking and Living With Cancer Study,” (in English). J Clin Oncol. 2023;41(2):295–306. https://doi.org/10.1200/JCO.22.00406.
La Carpia D, et al. “Cognitive decline in older long-term survivors from Non-Hodgkin Lymphoma: a multicenter cross-sectional study,” (in eng). J Geriatr Oncol. 2020;11(5):790–5. https://doi.org/10.1016/j.jgo.2020.01.007.
Ahles TA, et al. (2022) "The impact of APOE and smoking history on cognitive function in older, long-term breast cancer survivors." J Cancer Survivor. https://doi.org/10.1007/s11764-022-01267-z.
Rentscher KE, et al. “Epigenetic aging in older breast cancer survivors and noncancer controls: preliminary findings from the Thinking and Living with Cancer Study,” (in English). Cancer. 2023;129(17):2741–53. https://doi.org/10.1002/cncr.34818.
Kobayashi LC, et al. Cognitive function prior to systemic therapy and subsequent well-being in older breast cancer survivors: Longitudinal findings from the Thinking and Living with Cancer Study. Psychooncology. 2020;29(6):1051–9. https://doi.org/10.1002/pon.5376.
Wang K, Cheatham LP, Marbut AR, Chen X (2021) "Longitudinal associations between cancer history and cognitive functioning among older adults," (in English). Arch Gerontol Geriatr 97. https://doi.org/10.1016/j.archger.2021.104521.
Fowler ME, et al. (2022) "Longitudinal changes in patient-reported cognitive complaints among older adults with gastrointestinal malignancies - results from the Cancer and Aging Resilience Evaluation (CARE) Registry," (in English). J Cancer Survivor : Res Pract Article in Press 2022.https://doi.org/10.1007/s11764-022-01254-4.
Crouch A, Champion VL, Von Ah D. “Comorbidity, cognitive dysfunction, physical functioning, and quality of life in older breast cancer survivors,” (in English). Support Care Cancer. 2022;30(1):359–66. https://doi.org/10.1007/s00520-021-06427-y.
Delaney A, et al. “Progression of Frailty in Survivors of Childhood Cancer: A St. Jude Lifetime Cohort Report,” (in eng). J Natl Cancer Inst. 2021;113(10):1415–21. https://doi.org/10.1093/jnci/djab033.
Pranikoff S, et al. “Frail young adult cancer survivors experience poor health-related quality of life,” (in eng). Cancer. 2022;128(12):2375–83. https://doi.org/10.1002/cncr.34196.
Chipeeva N, Deviaterikova A, Glebova E, Romanova E, Karelin A, Kasatkin V (2022) "Comparison of Neurocognitive Functioning and Fine Motor Skills in Pediatric Cancer Survivors and Healthy Children," (in eng). Cancers (Basel) 14(23). https://doi.org/10.3390/cancers14235982.
Williams AM, et al. Physiologic Frailty and Neurocognitive Decline Among Young-Adult Childhood Cancer Survivors: A Prospective Study From the St Jude Lifetime Cohort. Journal of Clinical Oncology. 2021;39(31):3485-+. https://doi.org/10.1200/jco.21.00194.
Phillips NS, et al. “Late-onset Cognitive Impairment and Modifiable Risk Factors in Adult Childhood Cancer Survivors,” (in eng). JAMA Netw Open. 2023;6(5):e2316077. https://doi.org/10.1001/jamanetworkopen.2023.16077.
Kadan-Lottick NS, et al. “Patient-reported neurocognitive function in adult survivors of childhood and adolescent osteosarcoma and Ewing sarcoma,” (in eng). J Cancer Surviv. 2023;17(4):1238–50. https://doi.org/10.1007/s11764-021-01154-z.
Williams AM, et al. “Modifiable risk factors for neurocognitive and psychosocial problems after Hodgkin lymphoma,” (in English). Blood. 2022;139(20):3073–86. https://doi.org/10.1182/blood.2021013167.
Williams AM, et al. “Childhood Neurotoxicity and Brain Resilience to Adverse Events during Adulthood,” (in eng). Ann Neurol. 2021;89(3):534–45. https://doi.org/10.1002/ana.25981.
Stefanski KJ, et al. “Long-Term Neurocognitive and Psychosocial Outcomes After Acute Myeloid Leukemia: A Childhood Cancer Survivor Study Report,” (in eng). J Natl Cancer Inst. 2021;113(4):481–95. https://doi.org/10.1093/jnci/djaa102.
Tonning Olsson I, et al. “Neurocognitive and psychosocial outcomes in adult survivors of childhood soft-tissue sarcoma: A report from the St. Jude Lifetime Cohort,” (in eng). Cancer. 2020;126(7):1576–84. https://doi.org/10.1002/cncr.32694.
Tonning Olsson I, et al. “Neurocognitive outcomes in long-term survivors of Wilms tumor: a report from the St. Jude Lifetime Cohort,” (in eng). J Cancer Surviv. 2019;13(4):570–9. https://doi.org/10.1007/s11764-019-00776-8.
Hayek S, et al. Prevalence and Predictors of Frailty in Childhood Cancer Survivors and Siblings: A Report From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2020;38(3):232-+. https://doi.org/10.1200/jco.19.01226.
Atteveld JE, et al. Frailty and sarcopenia within the earliest national Dutch childhood cancer survivor cohort (DCCSS-LATER): a cross- sectional study. Lancet Healthy Longevity. 2023;4(4):E155–65 ([Online]. Available: <Go to ISI>://WOS:000976791500001).
Bhatt NS, et al. “Late outcomes in survivors of childhood acute myeloid leukemia: a report from the St. Jude Lifetime Cohort Study,” (in English). Leukemia. 2021;35(8):2258–73. https://doi.org/10.1038/s41375-021-01134-3.
Williams AM, et al. “Cognitive function in patients with chronic lymphocytic leukemia: a cross-sectional study examining effects of disease and treatment,” (in eng). Leuk Lymphoma. 2020;61(7):1627–35. https://doi.org/10.1080/10428194.2020.1728748.
Williams AM, et al. Premature aging as an accumulation of deficits in young adult survivors of pediatric cancer. Jnci-J Nat Cancer Inst. 2023;115(2):200–7. https://doi.org/10.1093/jnci/djac209.
Smitherman AB, et al. “Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty,” (in eng). Cancer. 2020;126(22):4975–83. https://doi.org/10.1002/cncr.33112.
Gehle SC, et al. “Accelerated epigenetic aging and myopenia in young adult cancer survivors,” (in eng). Cancer Med. 2023;12(11):12149–60. https://doi.org/10.1002/cam4.5908.
Avis NE, et al. “Health-related quality of life among breast cancer survivors and noncancer controls over 10 years: Pink SWAN,” (in eng). Cancer. 2020;126(10):2296–304. https://doi.org/10.1002/cncr.32757.
Michael YL, et al. “Postmenopausal Breast Cancer and Physical Function Change: A Difference-in-Differences Analysis,” (in eng). J Am Geriatr Soc. 2020;68(5):1029–36. https://doi.org/10.1111/jgs.16323.
Janelsins MC, et al. “Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study,” (in eng). J Clin Oncol. 2018;36(32):Jco2018786624. https://doi.org/10.1200/jco.2018.78.6624.
Alhareeri AA, et al. “Telomere lengths in women treated for breast cancer show associations with chemotherapy, pain symptoms, and cognitive domain measures: a longitudinal study,” (in eng). Breast Cancer Res. 2020;22(1):137. https://doi.org/10.1186/s13058-020-01368-6.
Magnuson A, et al. “Longitudinal Relationship Between Frailty and Cognition in Patients 50 Years and Older with Breast Cancer,” (in eng). J Am Geriatr Soc. 2019;67(5):928–36. https://doi.org/10.1111/jgs.15934.
Ji J, Sun CL, Cohen HJ, Muss HB, Bae M, Sedrak MS. “Toxicity risk score and clinical decline after adjuvant chemotherapy in older breast cancer survivors,” (in eng). J Natl Cancer Inst. 2023;115(5):578–85. https://doi.org/10.1093/jnci/djad029.
Yao S, et al. Impact of chemotherapy for breast cancer on leukocyte DNA methylation landscape and cognitive function: a prospective study. Clin Epigenet. 2019;11(1):45. https://doi.org/10.1186/s13148-019-0641-1.
Carroll JE, et al. “Cognitive performance in survivors of breast cancer and markers of biological aging,” (in English). Cancer. 2019;125(2):298–306. https://doi.org/10.1002/cncr.31777.
Gilmore N, et al. “Associations of inflammation with frailty in patients with breast cancer aged 50 and over receiving chemotherapy,” (in eng). J Geriatr Oncol. 2020;11(3):423–30. https://doi.org/10.1016/j.jgo.2019.04.001.
Belcher EK, et al. “Inflammation, Attention, and Processing Speed in Patients With Breast Cancer Before and After Chemotherapy,” (in eng). J Natl Cancer Inst. 2022;114(5):712–21. https://doi.org/10.1093/jnci/djac022.
Gilmore N, et al. The longitudinal relationship between immune cell profiles and frailty in patients with breast cancer receiving chemotherapy. Breast Cancer Res. 2021;23:1–11.
Van Der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB (2018) "Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study," (in English). Breast Cancer Res 20(1). https://doi.org/10.1186/s13058-018-1062-3.
Salerno EA, et al. “Physical Activity Patterns and Relationships With Cognitive Function in Patients With Breast Cancer Before, During, and After Chemotherapy in a Prospective, Nationwide Study,” (in English). J Clin Oncol. 2021;39(29):3283–92. https://doi.org/10.1200/JCO.20.03514.
Gregorowitsch ML, et al. “The effect of chemotherapy on subjective cognitive function in younger early-stage breast cancer survivors treated with chemotherapy compared to older patients,” (in English). Breast Cancer Res Treatment. 2019;175(2):429–41. https://doi.org/10.1007/s10549-019-05149-4.
Henneghan A, Haley AP, Kesler S. Exploring Relationships Among Peripheral Amyloid Beta, Tau, Cytokines, Cognitive Function, and Psychosomatic Symptoms in Breast Cancer Survivors. Biol Res Nurs. 2020;22(1):126–38. https://doi.org/10.1177/1099800419887230. (Art no. 1099800419887230).
Syarif H, Waluyo A, Afiyanti Y (2021) "Cognitive Perception among Post-Chemotherapy, Non-Chemotherapy Breast Cancer Survivors and Non-Cancer," (in English). Asian Pac J Cancer Prevent 22(6):1775–1780. https://doi.org/10.31557/APJCP.2021.22.6.1775.
Janelsins MC, et al. “Longitudinal Changes in Cognitive Function in a Nationwide Cohort Study of Patients With Lymphoma Treated With Chemotherapy,” (in eng). J Natl Cancer Inst. 2022;114(1):47–59. https://doi.org/10.1093/jnci/djab133.
Magyari F, et al. “Assessment of cognitive function in long-term Hodgkin lymphoma survivors, results based on data from a major treatment center in Hungary,” (in eng). Support Care Cancer. 2022;30(6):5249–58. https://doi.org/10.1007/s00520-022-06918-6.
Oh P-J, Moon SM. Changes of cognitive function and fatigue following chemotherapy in patients with gastrointestinal cancer: A prospective controlled study. Asian Oncol Nursing. 2019;19(3):126–34.
Sales MVC, et al. “Effects of Adjuvant Chemotherapy on Cognitive Function of Patients With Early-stage Colorectal Cancer,” (in eng). Clin Colorectal Cancer. 2019;18(1):19–27. https://doi.org/10.1016/j.clcc.2018.09.002.
Regier NG, et al. “Cancer-related cognitive impairment and associated factors in a sample of older male oral-digestive cancer survivors,” (in eng). Psychooncology. 2019;28(7):1551–8. https://doi.org/10.1002/pon.5131.
Vardy JL, et al. “Lack of cognitive impairment in long-term survivors of colorectal cancer,” (in English). Support Care Cancer. 2022;30(7):6123–33. https://doi.org/10.1007/s00520-022-07008-3.
Medysky ME, Dieckmann NF, Winters-Stone KM, Sullivan DR, Lyons KS. “Trajectories of Self-reported Physical Functioning and Symptoms in Lung Cancer Survivors,” (in eng). Cancer Nurs. 2021;44(2):E83-e89. https://doi.org/10.1097/ncc.0000000000000765.
Presley CJ, et al. “Functional Trajectories and Resilience Among Adults With Advanced Lung Cancer,” (in eng). JTO Clin Res Rep. 2022;3(6): 100334. https://doi.org/10.1016/j.jtocrr.2022.100334.
van der Willik KD, et al. “Change in cognition before and after non-central nervous system cancer diagnosis: A population-based cohort study,” (in eng). Psychooncology. 2021;30(10):1699–710. https://doi.org/10.1002/pon.5734.
De Rosa N, Della Corte L, Giannattasio A, Giampaolino P, Di Carlo C, Bifulco G. “Cancer-related cognitive impairment (CRCI), depression and quality of life in gynecological cancer patients: a prospective study,” (in English). Arch Gynecol Obstetr. 2021;303(6):1581–8. https://doi.org/10.1007/s00404-020-05896-6.
Cespedes Feliciano EM, et al. Long-term Trajectories of Physical Function Decline in Women With and Without Cancer. JAMA Oncol. 2023;9(3):395–403. https://doi.org/10.1001/jamaoncol.2022.6881.
Stelwagen J, et al. (2021) "Cognitive impairment in long‐term survivors of testicular cancer more than 20 years after treatment," (in English). Cancers 13(22). https://doi.org/10.3390/cancers13225675.
Brian DD, Melton LJ, Goellner JR, Williams RL, Ofallon WM. "Breast-cancer incidence, prevalence, mortality, and survivorship in Rochester, Minnesota, 1935 TO 1974." Mayo Clinic Proc 55(6):355–359. [Online]. Available: <Go to ISI>://WOS:A1980JV16600001.
Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35(5):815–21. https://doi.org/10.1188/08.Onf.815-821.
Wefel JS, Vardy J, Ahles T, Schagen SB. “International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer,” (in eng). Lancet Oncol. 2011;12(7):703–8. https://doi.org/10.1016/s1470-2045(10)70294-1.
Dale W, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J Clin Oncol. 2023;41(26):4293–312. https://doi.org/10.1200/jco.23.00933.
Acknowledgements
The authors would like to thank Jaimi McLean and Daniel Castillo (Health Sciences librarians, University of Rochester) for their help and advice with the search strategy of this scoping review. The authors also thank Susan Rosenthal, MD, for her editorial assistance and review of the manuscript.
Funding
This work was supported by the National Institute on Aging at the National Institute of Health R33 AG059206, K24AG056589 (Mohile), the National Cancer Institute K01CA276257 (Gilmore), F99CA284180 (Jensen-Battaglia), T32CA102618 (Yilmaz), R01CA231014 (Janelsins).
Author information
Authors and Affiliations
Contributions
Concept and design: Mostafa Mohamed, Supriya Mohile, Michelle Janelsins.
Collection and assembly of data: Mostafa Mohamed, Mustafa Ahmed.
Data analysis and interpretation: Mostafa Mohamed, Mostafa Ahmed, Supriya Mohile, Michelle Janelsins.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant conflicts of interest to report.
Consent for publication
This manuscript does not contain any individual person’s data in any form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supriya Mohile and Michelle Janelsins are co-senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, M., Ahmed, M., Williams, A.M. et al. A scoping review evaluating physical and cognitive functional outcomes in cancer survivors treated with chemotherapy: charting progress since the 2018 NCI think tank on cancer and aging phenotypes. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-024-01589-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-024-01589-0