Abstract
Purpose
Circadian rhythms control a wide range of physiological processes and may be associated with fatigue, depression, and sleep problems. We aimed to identify subgroups of breast cancer survivors based on symptoms of fatigue, insomnia, and depression; and assess whether circadian parameters (i.e., chronotype, amplitude, and stability) were associated with these subgroups over time.
Methods
Among breast cancer survivors, usual circadian parameters were assessed at 3–4 months after diagnosis (T0), and symptoms of fatigue, depression, and insomnia were assessed after 2–3 years (T1, N = 265) and 6–8 years (T2, N = 169). We applied latent class analysis to classify survivors in unobserved groups (“classes”) based on symptoms at T1. The impact of each of the circadian parameters on class allocation was assessed using multinomial logistic regression analysis, and changes in class allocation from T1 to T2 using latent transition models.
Results
We identified 3 latent classes of symptom burden: low (38%), moderate (41%), and high (21%). Survivors with a late chronotype (“evening types”) or low circadian amplitude (“languid types”) were more likely to have moderate or high symptom burden compared to “morning types” and “vigorous types,” respectively. The majority of survivors with moderate (59%) or high (64%) symptom burden at T1 had persistent symptom burden at T2.
Implications for Cancer Survivors
A late chronotype and lower circadian amplitude after breast cancer diagnosis were associated with greater symptoms of fatigue, depression, and insomnia at follow-up. These circadian parameters may potentially be novel targets in interventions aimed at alleviating symptom burden among breast cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer survivors report a multiplicity of symptoms that significantly and persistently impair their quality of life [1]. To date, symptoms in cancer survivors have mostly been studied in isolation, even though many symptoms share a common biological cause [2]. For instance, some behavioral symptoms, including fatigue, depression, and sleep problems, are likely triggered by common inflammatory and/or neuroendocrine responses to the cancer and/or treatments [3]. As a result, fatigue, depression, and insomnia often co-occur in the same individual; a phenomenon called symptom-clustering [4, 5]. Research into the underlying mechanisms of symptom-clustering is still in its infancy [6].
Misalignment of circadian (24-h) physiological processes, including hormone secretion, body temperature, and sleep–wake cycles, is a suspected risk factor for developing breast cancer [7]. The cancer itself [8] or breast cancer treatments [9] may cause further disruptions to circadian rhythms. Important parameters of the circadian rhythm are chronotype (i.e., preference for early or late wake- and bed-time), amplitude (i.e., ability to overcome drowsiness), and stability (i.e., affinity for routine sleeping) [10]. Compared with healthy controls, breast cancer survivors have relatively flattened 24-h production of the stress hormone cortisol with elevated levels in the evening [8, 11], a pattern that often results in a delayed circadian phase and sleep–wake cycle [12]. The impact of disrupted sleep schedules (i.e., due to shift-work) on sleepiness appears to be greater in individuals with low amplitude and high stability of their circadian cycles [13].
Evidence suggests that in healthy populations, individuals with a late chronotype (“evening type”), low amplitude (“languid type”), or instability (“flexible type”) of circadian cycles have increased risks of depression [14], fatigue [15], and poorer sleep quality and duration [13]. Therefore, circadian preference may influence the prevalence and clustering of these symptoms in breast cancer survivors. Insights into the underlying pathophysiological mechanisms of the depression, fatigue, and insomnia symptom cluster may provide directions for research to determine whether these circadian preferences can be potentially modified using psycho-oncological or pharmaceutical interventions such as timed bright light exposure [16] or melatonin supplements [17]. Therefore, in this study, we aimed to (1) identify groups of breast cancer survivors based on symptoms of fatigue, depression, and insomnia, and 2) assess whether circadian parameters (i.e., chronotype, amplitude, and stability) at baseline are associated with symptom burden at follow-up.
Methods
Design
The current study includes breast cancer survivors living in Western Australia (WA) who participated in the Breast Cancer Employment and Environment Study (BCEES) between 2009 and 2011 (referred to as “T0” henceforth) [18], and a subsequent study (Accurate Measurement of Physical Activity and Sedentary Time Among Breast Cancer Survivors Study; ACCEL-Breast) in 2013 [19] (referred to as “T1” henceforth) and 2017 (referred to as “T2” henceforth).
Population
For BCEES, all women diagnosed with a first incident invasive breast cancer between May 2009 and January 2011 were identified through the Western Australian Cancer Registry, of whom 2084 were deemed eligible (Supplementary file 1). Eligibility criteria included being female, 18 to 80 years of age and living in WA at the time of diagnosis, not having any serious other illness, and understanding English. From the 1205 women who completed the survey at T0 (57.8% response fraction), the 600 most recently diagnosed breast cancer survivors were invited to participate in the ACCEL-Breast study between April and December 2013 (T1). Survivors who participated at T1 were subsequently invited to complete a follow-up questionnaire between August and October 2017 (T2).
Measures
Clinical variables including time since diagnosis and cancer stage at diagnosis were derived from the Western Australian Cancer Registry. Menopausal status, smoking status, and BMI at diagnosis were self-reported at T0 and education, ethnicity, marital status, employment status, comorbidities, and cancer treatments received were self-reported in the follow-up questionnaire at T1.
Chronotype (i.e., morningness/eveningness), circadian amplitude (i.e., languidness/vigorousness), and circadian stability (i.e., flexibility/rigidity) were assessed at T0. Chronotype was assessed using the Horne-Ostberg Morningness/Eveningness scale [20], consisting of 14 items scored on a 4-point ordinal scale, such as follows: “How easy do you find it to get up in the morning?” (“very difficult” to “very easy”) and 5 items scored on along a continuum of timeslots, such as “What time would you get up/ go to bed if you were entirely free to plan you day?” (“5:00–6:30 AM” to “11AM–noon”). Total scores can range from 16 to 86, with lower scores indicating a higher degree of eveningness and higher scores indicating a higher degree of morningness. The internal consistency in our sample was good (Cronbach’s alpha = 0.77). Circadian amplitude and stability were assessed using the Circadian Type Inventory [21], consisting of 11 items scored on a 5-point Likert scale (“almost never” to “almost always”). The amplitude scale (5 items) ranges from 5 to 25, with lower scores indicating a higher degree of vigorousness and higher scores indicating a higher degree of languidness (Cronbach’s alpha = 0.73). The stability scale (6 items) ranges from 6 to 30, with lower scores indicating more rigid habits and higher scores indicating more flexibility (Cronbach’s alpha = 0.80). To obtain equal group sizes, survivors were categorized into tertiles, separately for each circadian parameter: chronotype (“morning type” (> 64), “neither type” (57–64), and “evening type” (< 57)); circadian amplitude (“vigorous type” (< 13), “neither type” (13–16), and “languid type” (> 16)); and circadian stability (“rigid type” (< 11), “neither type” (11–15), and “flexible type” (> 15)).
Symptoms of fatigue, insomnia, and depression were measured at T1 and T2. Fatigue was assessed using the 13-item FACIT-Fatigue [22]. Items were scored on a 5-point Likert scale (“not at all” to “very much”), with higher scores indicating more fatigue, except for two items that were reversed (“I have energy” and “I am able to do my usual activities”). Depression was measured with the 9-item Patient Health Questionnaire (PHQ9) [23]. Items were scored on a 4-point Likert scale (“not at all” to “nearly every day”), with higher scores indicating that more depression Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI) [24], consisting of 4 open questions about wake- and bed-time, hours of sleep, and minutes to fall asleep, and 14 questions scored on 4-point Likert scales with higher scores indicating more sleep problems. Seven component scores were calculated, as described previously [24].
Items from the PHQ9 that overlapped with the FACIT-Fatigue (“Trouble or falling/staying asleep or sleeping to much”) or PSQI (“Feeling tired or having little energy”) were excluded from analysis. The PSQI sleep efficiency scale was excluded because it is a function of the sleep duration and sleep efficiency scales. The three-item PSQI sleep dysfunction scale was also excluded due to overlap with items from the FACIT-Fatigue (“How often have you had trouble staying awake while driving, eating meals, or engaging in social activity?” and “How much of a problem has it been for you to keep up enough enthusiasm to get things done?”).
Statistical analysis
Latent class cluster analysis was conducted to identify unobserved (latent) groups based on symptoms of fatigue, depression, and sleep problems at T1. Latent class modeling is a data-driven approach, used to classify similar objects, with respect to a set of indicators, into groups [25, 26]. Based on the responses on these indicators, the model estimates posterior probabilities of class membership. That is, an individual can have a 80% probability of belonging to class 1 and 20% probability of belonging to class 2. Indicators used to define the classes were the FACIT-Fatigue and PHQ9 items and the PSQI component scales at T1. The optimal number of classes was derived from goodness-of-fit statistics and expert opinion on clinical relevance of the classes. Bivariate residuals were assessed to check if the local independency assumption was met. When bivariate residuals remain high with increasing number of classes in the model, the local independency assumption was relaxed. The model fit was assessed by differences in log-likelihood using bootstrapped p-values.
Sociodemographic and clinical characteristics were compared across the classes using bivariate analysis, with chi2 analyses for categorical variables and analysis of variance (ANOVA) for continuous variables. A generalized version of the weighted step-three approach was used as proposed by Bolck, Croon, and Hagenaars (2004) (BCH adjustment) [27]. Means and standard errors or percentages and standard errors were reported, with the p-values of the Wald test.
A multinomial logistic regression analyses was conducted to assess the associations of circadian parameters with the latent classes. Sociodemographic and clinical variables were entered as covariates and backward selection was performed to ensure control of significant covariates at p < 0.05. Means and standard errors, and odds ratios (OR) and 95% confidence intervals (CI) were reported, with p-values of the Wald test.
To assess changes in class allocation from T1 to T2, latent transition analysis was conducted [28]. Because of a considerable dropout of participants at follow-up, the measurement model of the latent transition analysis was estimated on data from T1 only and subsequently compared with the observed response patterns at T2. Based on these classifications, the transition probability from T1 to T2 was estimated. In addition, transition probabilities were compared between circadian types.
Analyses were conducted with Latent GOLD version 5.1 (Statistical Innovations Inc., Belmont, MA, USA).
Results
As previously reported, there were no meaningful or statistically significant differences between participants and non-participants of the ACCEL-Breast study for age, socio-economic status, time since diagnosis, or cancer grade [19]. Breast cancer survivors at T1 (N = 265) had an average age of 60 years and the majority were highly educated (trade/technical qualification or higher, 62.2%), Caucasian (92.5%), partnered (76.6%), and employed (52.1%). Most women had been diagnosed with stage I (44.5%) or II breast cancer (30.6%), and treated with surgery (98.9%), chemotherapy (50.4%), radiotherapy (66.7%), and/or hormonal therapy (74.2%) (Table 1). Participants at T2 (N = 168) were more often highly educated (trade/technical qualification or higher, 67.8 versus 53.0%, p < 0.01) compared with participants who dropped out after T1 (N = 102) (data not shown).
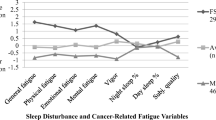
Using latent class analysis, we identified classes of survivors based on symptoms of fatigue (FACIT-Fatigue items), depression (PHQ9 items), and sleep problems (PSQI component scores). The local dependency assumption was relaxed for high bivariate residuals (sleep quality – sleep duration; sleep quality – sleep latency; sleep quality – sleep dysfunction). The 3-class model was selected based on the consistent Akaike’s information criterion (CAIC; Appendix Table 5) and interpretability of the classes, as additional classes were small (e.g., 5% of the sample in fourth class) and did not substantially differ from the other classes. Furthermore, an additional fourth class did not significantly improve model fit compared to the 3-class solution including bivariate residuals (-2LL diff. 14.3, p = 0.20). The final model included 3 classes: (1) low symptom burden (38.5%), 2) moderate symptom burden (40.7%) and high symptom burden (20.9%) (Fig. 1).
Compared to patients with low symptom burden, patients with moderate and high symptom burden were younger (58.5 and 58.2 vs. 62.7 years, p < 0.01), less likely to have a partner (75.5 and 63.8 vs. 84.7%, p = 0.02), more likely to work full-time (32.1 and 19.5 vs. 17.3%, p = 0.047), and were more likely to be obese (26.5 and 35.3 vs. 17.0%, p = 0.04) (Table 2).
After adjustment for covariates using backward selection (age and marital status), survivors with a late chronotype (“evening types”) were more likely to have moderate (OR = 3.38, 95% CI = 2.62–4.14, p < 0.01) or high (OR = 5.12, 95% CI = 4.16–6.08, p < 0.01) symptom burden compared to survivors with an early circadian phase (“morning types”). Furthermore, survivors with a moderate chronotype (“neither type”) were more likely to have a high symptom burden (OR = 2.11, 95% CI = 1.15–3.07, p < 0.05) compared to survivors with an early circadian phase (“morning type”). Survivors with a low circadian amplitude (“languid type”) or moderate circadian amplitude (“neither type”) were more likely to have moderate (ORlanguid = 2.44, 95% CI = 1.71–3.18, p < 0.01; ORneither = 2.02, 95% CI = 1.31–2.72, p < 0.01) or high (ORlanguid = 5.56, 95% CI = 4.64–6.49, p < 0.01; ORneither = 2.66, 95% CI = 1.72–3.60, p < 0.01) symptom burden compared to survivors with a high circadian amplitude (“vigorous type”) (Table 3).
Latent transition models showed that the majority of survivors in each class (80.3% low symptom burden; 58.6% moderate symptom burden; 63.6% high symptom burden) remained in their class at T2. However, 29.6% of survivors with moderate symptom burden and 34.5% of survivors with high symptom burden at T1 moved to low and moderate symptom burden classes respectively. Furthermore, 18.4% of survivors with low symptom burden and 11.9% of survivors with moderate symptom burden moved to moderate and high symptom burden classes respectively (Table 4).
A higher proportion of evening types than morning types had persistent symptom burden (persistent high 75 vs. 36%; persistent moderate 65 vs. 49%), and a lower proportion moved to a lower symptom burden class (high to moderate 24 vs. 59%; moderate to low 26 vs. 34%) (Appendix Table 6). A higher proportion of vigorous types than languid types had low symptom burden at T1, and a higher proportion of languid types than vigorous types had persistent low symptom burden at T2 (92 vs. 74%) (Appendix Table 7). Furthermore, transition probabilities were similar across circadian stability types except for rigid compared with flexible types with moderate symptom burden at T1, who showed a small trend towards the low symptom burden class (38 vs. 19%; Appendix Table 8).
Discussion
In this sample of breast cancer survivors, a high burden of symptoms of fatigue, depression, and sleep problems clustered in 21% of the population, while another 41% showed moderate levels of these symptoms, at 2–3 years after diagnosis. A late chronotype (“evening type”) and low circadian amplitude (“languid type”) after diagnosis were associated with a higher symptom burden after 2–3 years. The majority of survivors had persistent symptom burden after 6–8 years, with eveningness associated with lower symptom recovery.
The clustering of fatigue, depression, and sleep problems is consistent with previous studies in breast cancer survivors and other cancer populations [4]. However, sleep problems were prevalent across all identified symptom burden classes, with a PSQI global score above the clinical cut-off of 5 in each class [24]. Therefore, sleep problems were prevalent but did not cluster with depression and fatigue symptoms in the low symptom burden class. Interestingly, while previous results from our baseline data (BCEES) showed that survivors with a low circadian stability (“flexible type”) had a lower sleep duration [18], flexible types were not more likely to have a higher symptom burden in the current study. A possible explanation is that flexible types may cope better with sleep deprivation and be less vulnerable to poor sleep quality [13] and clustering with other symptoms. Associations with low circadian amplitude (“languid type”, i.e., the inability to overcome drowsiness) remain inconclusive; its similarity to fatigue meant that languidness was inevitably associated with high symptom burden. The similarity to fatigue may also explain why latent transition models suggested that languid types were more likely to experience persistent levels of symptom burden compared to vigorous types, and suggests that interventions to decrease overall symptom burden may be particularly beneficial for languid types.
Similar to studies in non-cancer populations [14, 15, 18], eveningness was associated with higher symptom burden. This has previously been explained by a dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis in evening types, as indicated by a decreased cortisol awakening response [12, 29]. According to the social jet lag theory, evening types show more misalignment of biological clock and social schedules, resulting in insufficient sleep, more fatigue, and more mental exhaustion [30]. However, symptoms of fatigue, depression, and sleep problems may in turn trigger dysregulation of the HPA axis [31]. Therefore, the causal relationships between circadian preference and these symptoms remained largely unclear using cross-sectional designs [14, 15, 18]. Albeit not statistically significant (due to small numbers), our transition models provide some evidence for eveningness being a risk factor of high symptom burden, a finding that merits further investigation.
While breast cancer treatments such as chemotherapy and radiotherapy have previously been found to trigger circadian dysregulation [9], neither treatment was a significant covariate in the relationship between circadian parameters and symptom burden in our analysis. Therefore, circadian preference in breast cancer survivors may be similar to that of the general population. Yet, even when circadian parameters are considered stable characteristics not influenced by the cancer and treatments, the identification of circadian types as potential risk factors may support targeted interventions to reduce long-term symptom burden.
Limitations
Although the three classes with different levels of overall symptom burden may provide evidence for a common, underlying clustering, our small sample size limited the possibility to identify additional subgroups of patients with more unique clustering of symptoms (i.e., depression symptoms but no fatigue or sleep problems). Importantly, symptom burden was measured only at follow-up, whereas circadian parameters were measured at diagnosis. Therefore, it is possible that symptom burden was already present at diagnosis and is not causally associated with circadian parameters. While our transition models provide some evidence for a causal relationship, further longitudinal or intervention research is warranted.
Only a selection of participants (i.e., the 600 most recently diagnosed) from the baseline study (BCEES) were selected for participation in the current study. Although there were no meaningful or statistical differences in sociodemographic or clinical variables between participants and non-participants at T1 [19], our study population was primarily Caucasian and well educated, particularly so for our sample at T2 due to selective drop-out. Although drop-out in survivorship research is common [32], our sample size and loss-to-follow-up (38%) resulted in limited statistical power of the transition models. Therefore, transition data were descriptive, not statistically tested, and should be interpreted with caution.
Furthermore, the overall symptom burden in our sample is relatively low compared to other cancer populations [22, 33] and morningness is relatively high [20, 34]. As a result, the tertile with highest eveningness in our sample may be misclassified as “evening type”, when compared to cutoffs that were validated in a student sample [20] or working population [34].
Future directions
This is the first study examining the overall burden of fatigue, depression, and sleep problems among cancer survivors in relation to circadian rhythm parameters. Whereas the types of cluster analysis that have traditionally been used in symptom cluster research (e.g., principal components analysis and common factor analysis using deterministic cluster assignment [5]) use deterministic cluster allocation, latent class analysis is a probabilistic statistical technique that accounts for uncertainty of cluster assignment and therefore prevents bias in our circadian parameter estimates. Furthermore, latent transition models provided the unique opportunity to assess transitions in symptom clustering over time. Despite the limitations of our small sample size, this study provides valuable new insights into the long-term associations of chronotype, circadian amplitude, and circadian stability with symptom burden in breast cancer survivors.
While associations with languidness remained inconclusive, eveningness was identified as a potential risk factor for long-term, co-occurring symptoms of fatigue, depression, and sleep problems in breast cancer survivors. Innate chronotype is largely influenced by non-modifiable factors including genetics and age; however, if the associations seen in our study are due to disruption of circadian rhythm in evening type women, it may be worth investigating interventions such as bright light exposure [16] and melatonin supplements [17] during or after breast cancer treatment which have shown promising effects on circadian re-alignment. Therefore, psycho-oncological or pharmaceutical therapies to re-align survivors’ chronotype and circadian amplitude could provide important directions to targeted interventions in evening types and may subsequently reduce long-term symptom burden. In future research, objective methods such as actigraphy and hormones including (24-h) cortisol and melatonin could provide more detailed information on chronotype for personalized therapies for re-alignment of chronotype and circadian amplitude [35, 36].
In conclusion, breast cancer survivors who are evening types may be at higher risk of accumulating symptoms of fatigue, depression, and sleep problems and may additionally be less likely to recover from these symptoms. Chronotype may be a novel target in interventions aimed at alleviating symptom burden among breast cancer survivors.
Data availability
The dataset cannot be shared due to ethical approval constraints.
References
Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Medicine. 2010;40(2):163–81.
Bower JE. Behavioral symptoms in breast cancer patients and survivors: fatigue, insomnia, depression, and cognitive disturbance. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26(5):768.
Kelly DL, Dickinson K, Hsiao C-P, Lukkahatai N, Gonzalez-Marrero V, McCabe M, Saligan LN. Biological basis for the clustering of symptoms. Semin Oncol Nurs. 2016;32(4):351–60.
Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23(2):127–35.
Barsevick A. Defining the symptom cluster: how far have we come? Seminars in Oncology Nursing. 2016;32(4):334-350.
Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, Kutner J, Patrick D, Zimmerman L, Xiao C, Matocha M, Marden S. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. JNCI: Journal of the National Cancer Institute. 2017;109(4);djw253.
He C, Anand ST, Ebell MH, Vena JE, Robb SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health. 2015;88(5):533–47.
Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082–92.
Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue biomed health behav. 2013;1(1–2):12–26.
Zee PC, Attarian H, Videnovic A. Circadian rhythm abnormalities. Contin: Lifelong Learn Neurol. 2013;19(1):132.
Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiat. 2003;54(3):269–82.
Randler C, Schaal S. Morningness–eveningness, habitual sleep-wake variables and cortisol level. Biol Psychol. 2010;85(1):14–8.
Jafari Roodbandi A, Choobineh A, Daneshvar S. Relationship between circadian rhythm amplitude and stability with sleep quality and sleepiness among shift nurses and health care workers. Int J Occup Saf Ergon. 2015;21(3):312–7.
Jeon HJ, Bang YR, Park HY, Kim SA, Yoon I-Y. Differential effects of circadian typology on sleep-related symptoms, physical fatigue and psychological well-being in relation to resilience. Chronobiol Int. 2017;34(6):677–86.
Antypa N, Verkuil B, Molendijk M, Schoevers R, Penninx BW, Van Der Does W. Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol Int. 2017;34(8):1125–35.
Neikrug AB, Rissling M, Trofimenko V, Liu L, Natarajan L, Lawton S, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10(3):202–16.
Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Reiter RJ. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin Investig Drugs. 2012;21(6):819–31.
Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am J Epidemiol. 2013;177(4):316–27.
Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181–90.
Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Folkard S. Circadian type inventory. Sheffield, United Kingdom: University of Sheffield, Department of Psychology; 1987.
Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–61.
Thekkumpurath P, Walker J, Butcher I, Hodges L, Kleiboer A, O’Connor M, et al. Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117(1):218–27.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Vermunt JK. Latent class modeling with covariates: two improved three-step approaches. Polit Anal. 2010;18(4):450–69.
McCutcheon AL. Latent class analysis (Sage University Paper series on Quantitative Applications in the Social Sciences, No. 07-064). 1987. Newbury Park, CA, Sage.
Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: One-step versus three-step estimators. Political Anal. 2004;12(1):3–27.
Bartolucci F, Montanari GE, Pandolfi S. Three-step estimation of latent Markov models with covariates. Comput Stat Data Anal. 2015;83:287–301.
Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–78.
Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509.
Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M, Consortium G. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–27.
Ramsey I, de Rooij BH, Mols F, Corsini N, Horevoorts NJ, Eckert M, et al. Cancer survivors who fully participate in the PROFILES registry have better health-related quality of life than those who drop out. J Cancer Surviv. 2019;13(6):829–39.
Hinz A, Mehnert A, Kocalevent R-D, Brähler E, Forkmann T, Singer S, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry. 2016;16(1):22.
Taillard J, Philip P, Chastang J-F, Bioulac B. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J Biol Rhythms. 2004;19(1):76–86.
Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405–15.
Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10(4):329–36.
Acknowledgements
We sincerely thank the people who took the time to participate in this study.
Funding
The ACCEL-Breast study was funded by a Project Grant from the Breast Cancer Research Centre—Western Australia. The case–control study (the Breast Cancer Environment and Employment Study) was funded by the Australian National Health and Medical Research Council (#573530). Lynch is supported by the Victorian Cancer Agency (MCRF-18005).
Author information
Authors and Affiliations
Contributions
Conceptualization: BR, TB, IR, NC.
Methodology: BR, TB, IR, NC, FC.
Formal analysis: BR, FC.
Investigation: TB, JV, BL, JH.
Data curation: BR, TB, IR, NC.
Writing – original draft: BR.
Writing – review and editing: BR, TB, IR, NC, JV, BL, JH.
Corresponding author
Ethics declarations
Ethics approval
Both the Breast Cancer Employment and Environment Study (BCEES) and a subsequent study (Accurate Measurement of Physical Activity and Sedentary Time Among Breast Cancer Survivors Study; ACCEL-Breast) were approved by the human ethics research committees of the Western Australian Department of Health and the University of Western Australia.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Rooij, B.H., Ramsey, I., Clouth, F.J. et al. The association of circadian parameters and the clustering of fatigue, depression, and sleep problems in breast cancer survivors: a latent class analysis. J Cancer Surviv 17, 1405–1415 (2023). https://doi.org/10.1007/s11764-022-01189-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-022-01189-w