Abstract
Purpose
Preexisting diabetes is associated with increased morbidity and mortality in cancer. We examined the impact of incident cancer on the long-term outcomes of diabetes.
Methods
Using the United Kingdom Clinical Practice Research Datalink, we identified three cohorts of diabetes patients subsequently diagnosed with breast, colorectal, or prostate cancer, each matched to diabetic noncancer controls. Patients were required to have survived at least 1 year after cancer diagnosis (cases) or a matched index date (controls), and were followed up to 10 years for incident microvascular and macrovascular complications and mortality. Multivariate competing risks regression analyses were used to compare outcomes between cancer patients and controls.
Results
Overall, there were 3382 cancer patients and 11,135 controls with 59,431 person-years of follow-up. In adjusted analyses, there were no statistically significant (p ≤ 0.05) differences in diabetes complication rates between cancer patients and their controls in any of the three cancer cohorts. Combined, cancer patients were less likely (adjusted hazard ratio [HR] 0.88; 95% CI = 0.79–0.98) to develop retinopathy. Cancer patients were more likely to die of any cause (including cancer), but prostate cancer patients were less likely to die of causes associated with diabetes (HR 0.61; 95% CI = 0.43–0.88).
Conclusions and implications
There is no evidence that incident cancer had an adverse impact on the long-term outcomes of preexisting diabetes.
Implications for Cancer Survivors
These findings are important for cancer survivors with preexisting diabetes because they suggest that substantial improvements in the relative survival of several of the most common types of cancer are not undermined by excess diabetes morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence from epidemiologic studies, as summarized in a number of recent reviews [1,2,3,4,5,6,7,8,9,10], indicates that preexisting diabetes is associated with worse short- and long-term outcomes of cancer, both overall [9, 10] and in specific types of cancer including breast [3, 4, 10], colorectal [2,3,4,5,6,7, 10], and prostate [8]. There are several reasons why the converse also may be true—that incident cancer adversely impacts the outcomes of preexisting diabetes. Potential mechanisms include adverse effects of cancer treatments on glycemic control [11, 12], impact of cancer on patient self-management of diabetes [13], and changes in the quality of diabetes primary care services during cancer treatment and follow-up [14,15,16,17,18,19]. However, there is considerably less information on how cancer impacts the long-term outcomes of preexisting diabetes [12, 20].
This gap in our understanding of how cancer impacts preexisting diabetes is important from several perspectives. For instance, as early detection and advances in cancer therapy and supportive care have substantially improved the relative survival of many of the most common types of cancer [21], overall morbidity and mortality in cancer depend increasingly on the quality and outcomes of primary care for other underlying conditions [22]. In response, cancer organizations, such as Cancer Research UK and Macmillan Cancer Support in the United Kingdom (UK), have expressed concern that overlooking other medical conditions during cancer treatment and follow-up could result in excess morbidity and mortality, thereby undermining gains associated with early detection and treatment of cancer [23, 24].
The objective of this study was to examine the effect of cancer on the long-term outcomes of preexisting diabetes.
Methods
Study design and data source
We conducted a historical cohort study using the UK Clinical Practice Research Datalink (CPRD) linked to the Office for National Statistics (ONS) mortality data. The CPRD contains anonymized information from general practitioner (GP) practices on demographics, symptoms, tests, diagnoses, therapies, health-related behaviors, and referrals to secondary care for over 11.3 million patients from 674 practices in the UK [25]. There are 4.4 million active (alive, currently registered) patients in the database, which is approximately 6.9% of the UK population. These patients are broadly representative of the UK general population in terms of age, sex, and ethnicity [25]. This database (including its predecessor, the General Practice Research Datalink) has been used extensively for health services and epidemiologic research in cancer and diabetes [26,27,28,29,30].
Patient selection
Using CPRD, we identified three cohorts of cancer patients with preexisting diabetes, each matched to diabetic noncancer controls. Cancer patients (cases) were included if they met all of the following criteria: (a) diagnosed with breast, colorectal, or prostate cancer on or after January 1st, 2000; (b) diagnosed with type I or type II diabetes at least 2 years before their date of cancer diagnosis (index date); (c) had no other cancer diagnosis, except nonmelanoma skin cancer, before their index date; (d) were age ≥ 50 years at their index date; (e) had at least 2 years of eligible CPRD data before their index date; (f) had an index date before the end of the eligible CPRD data; and (g) survived and were otherwise eligible for follow-up 1 year after cancer diagnosis. Patients were required to have been age ≥ 50 years at their index date because diabetes in older adults is linked to higher mortality [31] and because older adults with diabetes are at substantial risk for both acute and chronic microvascular and cardiovascular complications [32]. Men with breast cancer were excluded. Read codes from the Department of Health, Data and Business (QOF) Rules, Cancer and Diabetes Indicator Sets, version 25.0 [33], were used to identify diabetes and cancer in CPRD.
Control selection: matching
Each cancer patient (case) was matched to up to four noncancer patients (controls) with preexisting diabetes on GP practice number, sex (colorectal only), and age (±1 year) at cancer diagnosis. Matched controls were also required to have met inclusion criteria d–g above. In addition to the three cohorts of breast, colorectal, and prostate cancer patients plus noncancer controls, a fourth cohort was constructed by combining the three individual cohorts.
Patients were followed up to 10 years after their index date for new microvascular and macrovascular complications (as described below). They were followed from 1 year after their index date up to 10 years for all-cause and diabetes mortality.
Variables
Diabetes complications
Complications of diabetes consisted of incident microvascular and macrovascular conditions [34] first identified in patients’ electronic health records up to 10 years after their index date. Microvascular conditions consisted of retinopathy, neuropathy, nephropathy, chronic kidney disease (stage 4 or 5), and the composite outcome of any of the four above. Macrovascular complications consisted of peripheral arterial disease, acute myocardial infarction or coronary syndrome, cerebrovascular accident, lower limb amputation, and the composite outcome of any of the four above. Incident complications were identified using published lists of Read codes [35] present in patients’ records up to 10 years after their index date. Those patients identified with a specific complication prior to their index date were excluded from the population at risk for that complication during follow-up.
Mortality
Variables were constructed for all-cause and diabetes mortality, which was defined as the presence of an International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code in the ONS data indicating that the underlying cause of death was due to diabetes (ICD-10-CM E10–14), hyperglycemia (R73), hypoglycemia (E16.1, E16.2), myocardial infarction (I21–I22), ischemic heart disease (I20, I24, I25), stroke/sequelae (I60–I64, I69.0–I69.4), heart failure (I50), sudden death due to cardiac arrest (I46), peripheral vascular disease (I70–I74), or kidney disease (N00–N28) [36, 37].
Covariates
Baseline characteristics consisted of age at index date, sex, calendar year of index date, most recent (before the index date) smoking status, most recent drinking status, and index of multiple deprivation quintile, from least deprived (=1) to most deprived (=5); body mass index (kg/m2); and Charlson comorbidity index [38, 39]. Baseline clinical and laboratory values consisted of blood pressure (mm Hg), total cholesterol (mmol/L), and glycosylated hemoglobin (HbA1c: mmol/mol), identified with the use of the most recent value within 1 year before the index date. Categorical variables for laboratory values were constructed by using cutoffs that corresponded to the thresholds for meeting the laboratory-based performance indicators in the QOF Rules, Diabetes Indicator Set, version 25.0 [33]: blood pressure ≤140/80 mm Hg, total cholesterol ≤5 mmol/L (193 mg/dL), and HbA1c ≤59 (7.5%), 59–≤64 (8.0%), 64–≤75 (9.0%), and >75 mmol/mol. Baseline antidiabetic agents were identified with the use of the British National Formulary codes in the CPRD therapy file [40] within 1 year before the index date.
Statistical methods
Since there was a reasonable chance overall mortality would be higher in cancer patients than controls, we used competing risks regression according to the approach proposed by Fine and Gray [41] to estimate the cumulative incidence function and unadjusted and adjusted hazard ratios (HR) for each microvascular and macrovascular complication, comparing cancer patients to noncancer controls. Patients were censored at 10 years after their index date or at the end of their eligibility for follow-up in the data, whichever came first. The competing risk was death prior to the date of censoring. Adjusted analyses included baseline demographic and clinical covariates as described above. Patients with a specific diabetes complication prior to their index date were excluded from that particular analysis.
Competing risks regression [41] also was used to estimate unadjusted and adjusted HRs for all-cause and diabetes mortality. Only patients eligible for linkage to the ONS data were included in the mortality analyses. Also, since study inclusion criteria required patients to have survived at least 1 year after their index date, survival analyses included only years 2–10 of follow-up.
Results
Overall, there were 14,517 patients in the combined cohort: 3382 (23.3%) cancer patients and 11,135 (76.7%) controls (Table 1). There were no statistically significant differences in age, sex, and year of diagnosis between cancer patients and controls, because age and sex were used as matching criteria in constructing the cohorts, and controls received the same index date as their corresponding cases. There were small but statistically significant (p ≤ 0.05) differences between cancer patients and controls in the distributions of smoking status, BMI, and Charlson comorbidity index (Table 1). There were no differences between cancer patients and controls in mean baseline blood pressure or HbA1c. Cancer patients had statistically significantly lower mean baseline total cholesterol than controls (Table 1). However, the absolute difference was only 0.1 mmol/L (3.9 mg/dL).
The total number of years of follow-up in the combined cohort was 59,431, 13,372 (22.5%) of which were for cancer patients. Overall, the median length of follow-up was 1495 days (4.1 years), and follow-up was statistically significantly shorter in cancer patients (median 1444 days) than in controls (median 1511 days: log-rank test for equality of survivor functions, p < 0.0001).
Diabetes complications
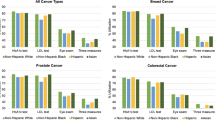
There were no differences between cancer patients and controls in the adjusted rate of any microvascular or macrovascular complication (Fig. 1), either in the combined cohort or in any of the three cancer cohorts (total of 80 unadjusted and adjusted comparisons), except that in the combined cohort and only in the colorectal cancer cohort, cancer patients were less likely to develop retinopathy (adjusted HRs 0.88; 95% CI = 0.79–0.98 and 0.81; 95% CI = 0.66–0.91, respectively). The results of sensitivity analyses in which a variable specifying the type of diabetes (type I or type II) was added to the list of predictors in the adjusted models were virtually identical to those reported above (results not shown) (Table 2).
Cumulative incidence of microvascular and macrovascular complications. Asterisk, microvascular complications consisted of retinopathy, neuropathy, nephropathy, or chronic kidney disease (stage 4 or 5). Macrovascular complications consisted of peripheral arterial disease, acute myocardial infarction or coronary syndrome, cerebrovascular accident, or lower limb amputation. Cancer patients (red) and controls (blue)
Mortality
All-cause mortality was statistically significantly higher in cancer patients than controls (Table 3). However, there was no evidence cancer adversely impacted diabetes-related mortality. Diabetes-related mortality was significantly lower among cancer patients in the combined cohort (adjusted HR 0.76; 95% CI = 0.61–0.94) and in the prostate cancer cohort (adjusted HR 0.61; 95% CI = 0.43–0.88) (Table 3). The results of sensitivity analyses in which a variable specifying the type of diabetes (type I or type II) was added to the list of predictors in the models were virtually identical to those reported above (results not shown).
Discussion
Evidence indicates that preexisting diabetes is associated with worse outcomes in several types of cancer. However, there is less information on whether incident cancer is associated with worse diabetes outcomes. This as an important gap because as the relative survival for many types of cancer continues to improve, overall survival depends increasingly on the quality and outcomes of care for other underlying conditions. Overall, this study suggests that in the UK, there is no reason to suspect that the outcomes of diabetes in the presence of cancer are adversely affected by the competing needs of cancer care.
Among 80 unadjusted and adjusted comparisons of diabetes complications, we found no instance in which cancer was associated with higher incidence of a complication. Cancer was associated with lower adjusted incidence of retinopathy in the colorectal cancer and the combined cohorts, but the reason for this is not immediately clear, and chance finding due to multiple testing cannot be ruled out. As might be expected, cancer was associated with higher all-cause mortality even though we excluded patients who survived less than 1 year. However, there was no evidence of an adverse impact on diabetes-related mortality. In fact, our findings suggest that prostate cancer was associated with lower diabetes mortality in competing risks regression that accounted for death due to other causes.
There are several possible reasons that we found no adverse associations between cancer and diabetes complications or mortality. First, we were able to adjust for differences in baseline characteristics between diabetic cancer patients and diabetic noncancer controls that otherwise could have confounded associations between cancer and diabetes outcomes. These included baseline BMI, smoking status, HbA1c, cholesterol, and blood pressure. Second, our study was conducted in the UK, which has a robust primary care system in which, under the quality and outcomes framework [33], there are financial incentives for GPs to provide high-quality diabetes care throughout the continuum of care for other conditions such as cancer.
Our study has several strengths, which are attributable largely to the high quality of the database we used. Notably, because the study was based on CPRD, we were able to adjust for additional clinical factors typically not available in US health insurance claims databases, which otherwise may have confounded associations between cancer and the outcomes of diabetes. These included baseline BMI, smoking status, blood pressure, cholesterol, and HbA1c. In addition, we had rich data about clinical outcomes over a relatively long period of time. Our study also has several limitations. First, at the time it was conducted, we were unable to link the CPRD data to information from the National Cancer Intelligence Network (NCIN), which would have given us details on cancer stage and initial treatment. Although primary care data have a high sensitivity and specificity for identifying cancer [42], registry data would have allowed us to exclude cancer patients diagnosed with metastatic disease. We considered using Read codes in the primary care data files or ICD-10 codes in the Hospital Episode Statistics (HES) inpatient data to stage patients. However, we are not aware of any studies in the UK that validate the use of ICD-10 codes for this purpose, and because only two thirds of the patients in our study were linked to HES, doing so would have limited our sample sizes for all the analyses. Instead, we excluded patients who died within the first year after their index date. Second, since we did not have access to cancer treatment data, we were unable to identify subgroups of cancer patients who may have been at higher risk of diabetes complications or related mortality due to the cancer treatment they received. This should be the subject of further research once linkage to NCIN and chemotherapy databases becomes more readily available. Third, although we required all patients to have survived at least 1 year after their index date, requiring longer survival might have enabled us to better assess the longer-term effects of cancer treatment among the cancer patients. Finally, our findings may not be generalizable to long-term survivors of breast, colorectal, and prostate cancer in an era of full implementation of QOF, which was designed to improve the quality of diabetes primary care, to other countries that do not have primary care performance measures for diabetes care in place, or to other types of cancers.
Conclusions
Overall, incident cancer appears to have had little adverse impact on the long-term outcomes of preexisting diabetes during 10 years after a diagnosis of breast, colorectal, or prostate cancer. These findings are important for cancer survivors with preexisting diabetes because they suggest that the advances in cancer therapy and supportive care, which have substantially improved the relative survival of several of the most common types of cancer, are not undermined by excess diabetes morbidity and mortality.
References
Giovannuci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85.
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56(11):1304–19.
De Bruijn KMJ, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CHJ. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–9.
Peairs KS, Barone BB, Snyder CF, Yeh H-C, Stein KB, Derr RL, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40–6.
Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–76.
Luo W, Cao Y, Liao C, Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Color Dis. 2011;14:1307–12.
Stein KB, Snyder CF, Barone BB, Yeh H-C, Peairs KS, Derr RL, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:1839–51.
Snyder CF, Stein KB, Barone BB, Peairs KS, Yeh H-C, Derr RL, et al. Does pre-existing diabetes affect prostate cancer prognosis? A systematic review. Prostate Cancer Prostatic Dis. 2010;13:58–64.
Barone BB, Yeh H-C, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33(4):931–9.
Barone BB, Yeh H-C, Snyder CF, Pearis KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–64.
Psarakis HM. Clinical challenges in caring for patients with diabetes and cancer. Diabetes Spectrum. 2006;19(3):157–62.
Keating NL, Liu P-H, O’Malley AJ, Freedland SJ, Smith MR. Androgen deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol. 2014;65(4):816–24.
Hershey DS, Tipton J, Given B, Davis E. Perceived impact of cancer treatment on diabetes self-management. The Diabetes Educator. 2012;38(6):779–90.
Snyder CF, Frick KD, Herbert RJ, Blackford AL, Neville BA, Wolff AC, et al. Quality of care for comorbid conditions during the transition to survivorship: differences between cancer survivors and noncancer controls. J Clin Oncol. 2013;31(9):1140–8.
Khan NF, Mant D, Rose PW. Quality of care for chronic diseases in a British cohort of long-term cancer survivors. Ann Fam Med. 2011;8(5):418–24.
Chiao EY, Nambi PV, Naik AD. The impact of diabetes process and outcome quality measures on overall survival in patients with co-morbid colorectal cancer. J Cancer Surviv. 2010;4(4):381–7.
Hanchate AD, Clough-Gorr KM, Ash AS, Thwin SS, Silliman RA. Longitudinal patterns in survival, comorbidity, healthcare utilization and quality of care among older women following breast cancer diagnosis. J Gen Intern Med. 2010;25(10):1045–50.
Keating NL, Zaslavsky AM, Herrinton LJ, Selby JV, Wolf RE, Ayanian JZ. Quality of diabetes care among cancer survivors with diabetes. Med Care. 2007;45(9):869–75.
Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–9.
Bayliss EA, Blatchford PJ, Newcomer SR, Steiner JF, Fairclough DL. The effect of incident cancer, depression and pulmonary disease exacerbations on type 2 diabetes control. J Gen Intern Med. 2011;26(6):575–81.
Cancer Research UK. Cancer survival for common cancers. http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival/common-cancers-compared#undefined. Accessed Feb 2016.
Ning Yi, Shen Qin, Herrick K, Mikkelsen R, Anscher M, Houlihan R, Lapane K. Cause of death in cancer survivors. Cancer Res 2012;72(8 Suppl):Abstract nr LB-339.
Cancer Research UK. Longer cancer survival means nearly half of cancer patients die from other diseases. http://www.cancerresearchuk.org/about-us/cancer-news/news-report/2012-04-03-longer-cancer-survival-means-nearly-half-of-cancer-patients-die-from-other-diseases?view=rss. Accessed Feb 2016.
Macmillan Cancer Support. Throwing light on the consequences of cancer and its treatment. July 2013. http://www.ncsi.org.uk/wp-content/uploads/MAC14312_CoT_Throwing-light_report_FINAL.pdf. Accessed Feb 2016
Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36.
Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105:S29–37.
Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care. 2013;36(8):2366–71.
Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731.
Redaniel MTM, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer Causes Control. 2012;23:1785–95.
Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61(582):e12–21.
Brown AF, Mangione CM, Saliba D, Sarkisian CA. Healthcare Foundation/American Geriatrics Society panel on improving care for elders with diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(Suppl. Guidelines):S265–80.
Kirkman MS, Jones Briscoe V, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–64.
Primary Care Commissioning (PCC). QOF business rules v25. http://www.pcc-cic.org.uk/article/qof-business-rules-v25.0. Accessed Feb 2016.
Adler AI, Stratton IM, Neil HAW, Yudkin JS, Matthews DR, Cull CA, et al., on behalf of the UK Prospective Diabetes Study Group. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9.
Kontopantelis E, Springate DA, Reeves D, Ashcroft DM, Rutter M, Buchan I, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia. 2015;58(3):505–18.
Australian Institute of Health and Welfare. http://www.aihw.gov.au/diabetes-indicators/deaths/. Accessed 19 Jan 2016.
Ricci-Cabello I, Stevens S, Kontopantelis E, Dalton ARH, Griffiths RI, Campbell JL, et al. Impact of the prevalence of concordant and discordant conditions on the quality of diabetes care in family practices in England. Ann Fam Med. 2015;13:514–22.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010:11(1).
Dave S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18(8):704–7.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Boggon R, van Staa TP, Chapman M, Gallagher AM, Hammad TA, Richards MA. Cancer recording and mortality in the General Practice Research Database and linked cancer registries. Pharmacoepidemiol Drug Saf. 2013;22:168–75.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the work. RIG and ECM acquired the data. RIG and ECM performed the analyses. All authors contributed to the interpretation of the data for the work. RIG drafted the manuscript. All authors contributed to revising it critically for important intellectual content. RIG takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
Funding
This study was funded by the Population Research Committee, Cancer Research UK. Quality and Outcomes of Care for Chronic Conditions in Older Patients Diagnosed with Breast, Colorectal, or Prostate Cancer Compared to Non-Cancer Controls: An Observational Study Using the Clinical Practice Research Datalink (CPRD). Reference # 16609. 1 July 2013–29 February, 2016. In addition, Dr. Keating is supported by K24CA181510 from the US National Cancer Institute.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The protocol for this study was approved by the Independent Scientific Advisory Committee (ISAC) to the CPRD on 1 August 2013 (ISAC reference number 13_124) with subsequent amendments approved by ISAC on 22 May 2014. Changes relevant to the analyses presented here include identification of diabetes complications through the primary care record, as described above, rather than from hospital data; this change was made because hospital data linkage was not available in all patients. The ISAC protocol was made available to the reviewers and editors during the peer-review process.
Additional information
Richard J. Stevens and Nancy L. Keating are co-senior authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Griffiths, R.I., Valderas, J.M., McFadden, E.C. et al. Outcomes of preexisting diabetes mellitus in breast, colorectal, and prostate cancer. J Cancer Surviv 11, 604–613 (2017). https://doi.org/10.1007/s11764-017-0631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0631-2