Abstract
Tenebrio molitor gained recognition for its nutritional value for human and/or animal consumption, its utilization as a model species in research studies, and its ability to degrade many types of plastics. However, it is a stored-product pest infesting a wide range of commodities in storages. The impact of temperature on the development of T. molitor was evaluated, taking into account confounding effects of other covariates such as developmental stage and larval instar. The time for larval development was longer at 22.5 °C than at 25 °C, 27.5 °C, and 30 °C. Tenebrio molitor spent most of its lifetime as larva > egg, pupa, since the outcome of our model inference resulted that the estimated probability of longer duration in larvae is approximately 25.5 times higher compared to eggs and pupae, and passed through 26 instars before it became pupa. Duration of larval instars increased significantly up to L22 and then decreased until L26. Among larval instars, L22 exhibited the highest developmental duration while the lowest was recorded for L1. These findings enhance the knowledge about the biology of T. molitor, leading to precise decisions for its successful culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tenebrio molitor L., 1758 (Coleoptera: Tenebrionidae) is a famous edible insect (Bußler et al. 2016; Azzollini et al. 2018; Costa et al. 2020; Cho and Ryu 2021; Djouadi et al. 2022; Sriprablom et al. 2022). It can be consumed in many forms, i.e., cereal snacks, crackers, cookies (Azzollini et al. 2018; Djouadi et al. 2022; Sriprablom et al. 2022). The addition of T. molitor in food products enhances some of their characteristics like crispness (Djouadi et al. 2022) or their nutritional value (Bußler et al. 2016; Cho and Ryu 2021; Sriprablom et al. 2022). In a recent study, insect powder from T. molitor was introduced in cereal snacks, regulating their digestibility (Azzollini et al. 2018). In addition, when dry basis of T. molitor was mixed with soy-based meat analog, it enhanced the taste and the antioxidant activity of the final product (Cho and Ryu 2021). The high protein content of T. molitor is suitable as an intermediate product for feed and food improvement (Bußler et al. 2016). One other use of T. molitor (usually larvae) is as feed for reptiles (Hill 2003; Mouadi et al. 2022), crustaceans (Mazlum et al. 2021), birds (Hill 2003; Shaviklo et al. 2021), and fish (Shafique et al. 2021).
Interestingly, there is advanced knowledge of the physiology and immunology of T. molitor (Vigneron et al. 2019; Vommaro et al. 2021) allowing it to be used as a model in host–pathogen relationships (De Souza et al. 2015; Lozoya-Pérez et al. 2021). Recently, studies have revealed additional interesting properties of T. molitor that can potentially be used in food and pharmaceutical industry, biomedicine, and environmental research (Bußler et al. 2016; Adamski et al. 2019; Chen et al. 2019; Ding et al. 2021; Hwang et al. 2022). For example, the immunized larval extract of T. molitor has antimicrobial properties against the bacteria Staphylococcus aureus Rosenbach, 1884 (Bacillales: Staphylococcaceae), Bacillus cereus Frankland and Frankland, 1887 (Bacillales: Bacillaceae), and Escherichia coli (Migula, 1895) Castellani and Chalmers, 1919 (Enterobacterales: Enterobacteriaceae), and the fungi Wickerhamomyces anomalus (E.C. Hansen) Kurtzman, Robnett and Basehoar-Powers, 2008 (Saccharomycetales: Saccharomycetaceae), Aspergillus parasiticus Speare, 1912 (Eurotiales: Trichocomaceae), and Aspergillus flavus Link, 1809 (Eurotiales: Trichocomaceae) (Hwang et al. 2022). Thus, this extract can be used by the food industry as a natural preservative, prolonging the life of the product (Hwang et al. 2022). Chen et al. (2019) reported the antithrombotic activity of proteins found on T. molitor larvae powder that potentially can be developed to antithrombotic drugs. It should be noted that aqueous extracts from T. molitor larvae exhibited antiproliferative activity against human colorectal adenocarcinoma and hepatocellular carcinoma (Ding et al. 2021). Protein gels from T. molitor can be used as substitutes for ointments to heal wounds (Klost et al. 2022). Furthermore, antifreeze proteins extracted from T. molitor larvae preserved the cell-structure of many vegetables like zucchini, carrot, onion, and cucumber (Song et al. 2019).
Tenebrio molitor has elevated properties in degradation of several types of plastic. Recently, Espinoza-Pinchi et al. (2022) found that larvae caused the biodegradation of polystyrene, polyethylene terephthalate, and polyvinyl chloride microplastics. Sugumar et al. (2022) reported that plastics, waste papers, and polysterenes are biodegradated by larvae due to their enzymes and gut microorganisms. Interestingly, larvae did not die after the consumption of the aforementioned substances (Sugumar et al. 2022). Larvae of T. molitor can also biodegrade polylactic acid (Peng et al. 2021). Even after 25 days of consuming 100% polylactic acid, the survival rate of individuals was ~ 83% (Peng et al. 2021). Yang et al. (2018) also tracked digestive enzymes of T. molitor, apart from gut microorganisms, that depolymerized low-density polyethylene. It should be noted that temperature has a crucial role at the biodegradation of plastics since T. molitor larval polystyrene consumption differed significantly when it was conducted at 20, 25, and 30 °C (Yang et al. 2021).
Apart from its beneficial uses, T. molitor is a secondary stored-product pest, infesting over 46 plant and animal commodities at storages, farms, grain elevators, or even bakeries (Hagstrum and Subramanyam 2009). It has global distribution, ability to fly, and can live for more than two years (Rees 2004; Robinson 2005). Even though the biology of T. molitor, in terms of temperature has been searched, the available information focuses on the optimal condition for mass rearing and population growth (Bjørge et al. 2018; Adámková et al. 2020; Deruytter et al. 2022; Eberle et al. 2022). For instance, Bjørge et al. (2018) studied the mass growth rate and the lipid/water/protein content of T. molitor at different temperatures. Adámková et al. (2020) reported the optimal temperatures to achieve the highest crude protein content and fat content of T. molitor. Deruytter et al. (2022) noticed the average weight and the biomass of T. molitor at various temperatures. In a recent study, Eberle et al. (2022) studied the effect of temperature on the larval weight, survival, and development of T. molitor. Among the three tested temperatures, 25 and 30 °C were more favorable than 20 °C, while authors indicated than the optimum temperature lies between 25 and 30 °C. However, there are no data about the duration of T. molitor per immature stage and larval instar in relation to temperature. Thus, the objective of this study was to assess the effect of temperature and other explanatory variables (i.e., stage, larval instar) on the developmental duration of T. molitor. For this purpose, a predictive statistical modeling approach was taken, utilizing a Poisson log-linear regression model. This model uses a log transformation as the link function between days of developmental duration of T. molitor and the linear function of explanatory variables. These days are assumed as collected data to generate a population that follows a Poisson distribution since the response variable takes only integer non-negative values.
Materials and methods
Insects and food
Tenebrio molitor was reared on 100% oat bran plus potato slices (for humidity enhancement) at 30 °C, 65% relative humidity (RH), and lack of light source (Kavallieratos et al. 2024). The initial insects were obtained from Greek warehouses in 2014 and have since been bred at the Laboratory of Agricultural Zoology and Entomology (Agricultural University of Athens). For the experiment, oat bran was used as feed.
Bioassays
The trial procedure started by placing 2 g of pre-sieved oat bran separately in each 125 mm height × 75 mm diameter glass vials (Krassas, Athens, Greece). The feed was weighed using the Precisa XB3200D compact scale (Alpha Analytical Instruments, Greece). The mini GAC plus moisture meter (Dickey-John Europe S.A.S., France) was used to measure the moisture content, i.e., 13.7%. A single circular hole (15 mm diameter) was previously drilled in the center of the lids of the vials, to adequately ventilate their interiors and covered with muslin. As an additional protection against the escape of insects from the vials, their upper inner wall was covered with polytetrafluoroethylene (60% by weight dispersion in water) of the commercial formulation of Sigma-Aldrich Chemie GmbH (Germany). The eggs used in the experiment were produced by 50 T. molitor adults ~ 7 days old. These adults had been transferred to a 4-L glass jar containing 500 ml of pre-sifted oat bran for one day. Eggs and adults were then removed from the oat bran using two U.S. standard testing sieves: No 20 (0.85 mm openings) and No 60 (0.25 mm openings) (Advantech Manufacturing Inc., USA). The remaining eggs on the surface of a sieve No. 60 were carefully moved with a fine brush individually to separate Petri dishes (10 mm height × 55 mm diameter) (WhiteSci, Cape Town, South Africa) without oat bran. Then, dishes were placed inside incubators at 22.5 °C and 65% RH in order to record their duration daily using a SZX9 Olympus stereoscopic microscope (Bacacos S.A., Greece) of total 57 × magnification. Eggs that appeared black were recorded as dead. Eggs were inspected daily to record the duration. The newly emerged larval individuals were separately placed through a fine brush in the prepared vials (see above). The vials were placed at the tested abiotic conditions (22.5 °C, 65% RH, without light). The duration of larvae and pupae were recorded daily. Larvae were recorded as dead if they were dehydrated, turned to brown, and/or they didn't make any movement. Exuviae were removed from vials. Pupae were considered dead if they were dehydrated and/or turned to brown. The diet in vials was replaced with new diet monthly. The above procedure was followed for 25 °C, 27.5 °C, and 30 °C and 65% RH using new adults (50 individuals), jars and oat bran to receive the eggs and later the larvae. For the entire experimental procedure, 200 eggs were used (50 eggs for each temperature). The temperatures which were implemented in the experiment fall within the range for the growth of T. molitor (> 20 °C, ≤ 30 °C), as suggested by Eberle et al. (2022).
Statistical analysis
A Poisson log-linear regression modeling approach was followed to examine the potential effects on the developmental duration in days of T. molitor of several covariates. These covariates include the variable of temperature, utilized as a categorical variable consisting of four levels (22.5 °C, 25 °C, 27.5 °C, 30 °C), along with two distinct covariates, i.e., stage and larval instar of T. molitor. The two covariates are also categorical, with stage consisting of three levels (pupa, larva, and egg) and larval instar consisting of 26 levels (L1 to L26). The distribution of response data (developmental duration days) has been tested via Kolmogorov–Smirnov test and Poisson distribution was not rejected at a 1% significance level. Hence, a Poisson log-linear regression model was opted, including developmental duration in days as a response variable due to the specific nature of the response variable, being counts data. Specifically, two different regression-type models (A and B) were fitted, where temperature is present in both models, and separately examined the effects of each one of the remaining two biotic covariates (stage and larval instar). Each biotic covariate was examined separately in relation to temperature because the biotic covariate larval instar is further segmented to more sublevels (multiple larval instars). The insect should pass through different numerous larval instars to become pupa and finally adult. A Poisson log-linear regression model (Greene 2003) is fitted to the data to evaluate the differences in the developmental duration of T. molitor for the various levels of the covariates. The theoretical equations of two models are presented below:
Model A
or
Model B
where \({Y}_{i} \sim Poisson \left({\lambda }_{i}\right)\) are the i responses (i = 1,2,…,n) of the developmental duration in days variable, that are assumed to follow a Poisson distribution with mean λi since the variable takes only integer values (n is 601, 2701 for the stage and larval instar models, respectively). As an independent variable, besides temperature, the first model includes the categorical variable of three stages (pupa, larva, egg), with reference category being the level of “pupa”. The second model utilizes the “larval instar” covariate, consisting of 26 different levels in total, with L1 used as the reference category.
Finally, β0 is the intercept, and \({\beta }_{j}\left(j=1, \dots , m\right)\) are the regression coefficients of the categorical explanatory variables in the two models (m = 5 and 28, respectively). The error term ε is utilized to measure the unexplained variance in dependent variables due to covariates and is distributed as a Gaussian random variable with zero mean and constant variance σ2.
The R software (The R Project for Statistical Computing 2023) has been utilized for fitting the Poisson regression models. In particular, for model fitting and inference for the estimation of regression coefficients, \(\widehat{\beta }=b\), the glm() function we used, that was suitable for fitting models of the general linear model family, including the Poisson log-linear regression model. Model fit for the two regression-type models was assessed through the Akaike Information Criterion (AIC) and visual inspections via scatterplots of observed versus fitted values of the response variables.
Results
Descriptive analysis
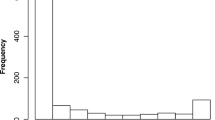
The descriptive statistics for the variables involved in regression analysis are presented. The frequency distribution for the number of the developmental duration in days of T. molitor for the stage and larval instar data are shown in the frequency charts. Duration days are concentrated between zero and 50 (stage dataset) (Fig. 1a) and between zero and 20 (larval instar dataset) (Fig. 1b). The corresponding descriptive measures for developmental duration in days are presented in Table 1.
The boxplots of number of days of duration of T. molitor between the different rearing temperatures are shown in Fig. 2. While the median duration of the sample collected is relatively similar for all temperature levels, and interquartiles are higher for 25 °C and 30 °C, the presence of a relatively large number of durations around 400 days (18 samples exceed a duration higher than 300 days especially for 22.5 °C) increases significantly the average duration at this temperature level.
The Fig. 3 presents in more detail the duration of life stages, as the former is summarized by histogram plots at the different temperature levels. As it is evidently shown in the graphs of Fig. 3, the stage that is mostly influenced by temperature in terms of duration is larva.
Poisson log-linear regression results
Poisson log-linear model including “Stage” covariate
Table 2 presents the results of the Poisson log-linear regression model for the stage data (Model A), where the independent variables are the categorical variables of temperature and stage. Both covariates are statistically significant for the variation in the response variable. Regarding temperature levels, an increase results in a reduction of the duration of T. molitor since the estimated beta coefficients of the three levels of temperature (25 °C, 27.5 °C, 30 °C) are all negative and differ significantly from the reference category of 22.5 °C.
When considering the covariate of stage, the basic outcome is that the duration of T. molitor is higher for the [larva] stage (beta = 3.240; p < 0.001). On the other hand, the duration of T. molitor is at its lowest levels for the stages of [pupa] and [egg] (beta coefficient = -0.016 for egg). According to these estimates, the probability of higher duration in larvae is approximately 25.5 times higher compared to eggs and pupae [probability = exp(3.24)≈25.5]. Another verification of the above results is shown in Table 2 by the 95% confidence intervals of the two stages when compared to the reference category of pupa. Since the confidence intervals for [pupa] and [egg] do not differ statistically significantly, it may be deduced that there is no significant difference in the effect of the latter on the duration of T. molitor.
Finally, when examining the fit of the best selected Poisson log-linear model (Fig. 4), the scatterplot of observed vs. fitted response reveals that the best fit is achieved for the lower values of the response, whereas the fit is less accurate for the higher values.
Poisson model including “Larval instar” covariate
Table 3 shows the parameter estimate results for the log-linear regression model that involves as independent variables the abiotic variable of temperature and the “larval instar” covariate. The estimates show (as in the previous model) that increased temperature results in a reduction of the duration of T. molitor. The lowest duration of T. molitor falls at 30 °C (beta = -0.535, p < 0.001). Table 3 also shows the 95% confidence intervals for the temperature and instar covariates for the “larval instar” model. For a visual representation of the confidence intervals we also include Fig. 5. Concerning the effects of “larval instar” covariate of T. molitor on its duration, the estimation of the corresponding parameters for each level of the larval instar covariate shows the significant difference of instar L2 to L26 with the reference category of L1 (Table 3).
On the basis of 95% confidence intervals of the parameter estimates of the “larval instar” covariate, it is revealed that the duration of T. molitor increases as the larval instar increases, up to a certain point, after which the duration reduces (Fig. 5). Specifically, it appears that duration is approximately at the same level for larval instars ranging between L2 and L10. Duration increases significantly (with a slight reduction at L20) up to L22 (Fig. 5). Then, the duration decreases slightly until the last larval instar (L26). Up to L23, uncertainty in the estimation of parameters for each larval instar is much reduced when compared to uncertainty of larval instars between L24 and L26. Therefore, duration in the latter larval instar is more variable in comparison to previous larval instars.
Model fit of the larval instar model was assessed through the AIC estimate (AIC = 15,735) and visual comparison of observed versus predictions for the response variable (Fig. 6). Comparisons of the log-linear Poisson model for the larval instar data with the respective model for the stage data is indicative for a better fit of the former model. This is verified from both the comparison of AIC values and the inspection of scatterplots of the observed and fitted values of the responses.
Discussion
This is the first application of a log-linear Poisson model for assessing the effects of temperature on the development of T. molitor. Previous studies have examined the impact on temperature on several aspects of T. molitor development and nutritional value by utilizing analysis of variance (ANOVA) (Bjørge et al. 2018; Adámková et al. 2020; Deruytter et al. 2022; Eberle et al. 2022). Our analysis, where the response variable is a count of events, however, naturally proposes the use of a non-linear extension of the typical linear regression model, such as the Poisson log-linear model that effectively allows for non-linear relationships to be captured (Greene 2003).
Here, it is indicated the crucial role of temperature in the developmental duration of T. molitor. Generally, when T. molitor immature stages grew at 22.5 °C exhibited increased developmental duration than at 25, 27.5, and 30 °C. Recently, Eberle et al. (2022) found that T. molitor exhibited the longest developmental time at 20 °C. Also, Adámková et al. (2017) revealed that fat content of T. molitor increased from 17 °C (14.67%) to 23 °C (24.56%) when breeding took place at these temperatures. A further temperature increase to 28 °C led to a decrease in fat content (23.32%). Interestingly, the shortest overall duration of T. molitor was noted at 27.5 °C, but it increased when the temperature rose to 30 °C, without significant difference. Similarly, Trogoderma granarium Everts, 1898 (Coleoptera: Dermestidae) showed the longest development at 30 °C, followed by 35 and 40 °C (Papanikolaou et al. 2019). For Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae), the shortest duration was observed at 30 °C and increased at 20, 25, and 32.5 °C (Skourti et al. 2019). Concerning Oryzaephilus surinamensis (L., 1758) (Coleoptera: Silvanidae), the development of immature period lasted less at 32.5 °C than at 20, 25, 30, and 35 °C (Nika et al. 2021). Even strains of the same stored-product insect exhibit differences when are developed under different temperatures. For instance, between two Tribolium confusum Jacquelin du Val, 1863 (Coleoptera: Tenebrionidae) geographical strains, the Greek strain had longer immature duration than the Serbian strain at 25, 30, and 32.5 °C (Kavallieratos et al. 2022). Given the diversity of the aforementioned results, we can conclude that each stored-product species/ strain has a different range of temperatures that favor the development and consequently the damage potential. This is rather expected since insects are ectotherms and therefore temperature affects their biological processes, developmental period, weight, and phenotype (Honěk 1996; Sönmez and Koç 2019; Nika et al. 2021). The fact that stored-product insects co-exist in the same environment affects the damage they can cause to grain commodities (Athanassiou et al. 2017; Kavallieratos et al. 2017; Papanikolaou et al. 2018; Nika et al. 2022a, b). The differences in temperature requirements could favor some species over others altering competition for food resources. For instance, the population growth of Liposcelis bostrychophila Badonnel, 1931 (Psocoptera: Liposcelididae) was reduced under the simultaneous presence of Liposcelis decolor (Pearman, 1925) or Liposcelis paeta Pearman, 1942 (Psocoptera: Liposcelididae) at 30 °C vs. 25 °C (Athanassiou et al. 2014). The increase of temperature from 25 °C to 30 °C and 35 °C resulted to the rapid increase of the numbers of T. granarium larvae while the numbers of Sitophilus oryzae (L., 1763) (Coleoptera: Curculionidae) or Rhyzopertha dominica (F., 1792) (Coleoptera: Bostrychidae) were considerably reduced (Kavallieratos et al. 2017). The plasticity to regulate the speed of the life cycle would reduce the problems related to conspecific and interspecific competition. This information would be important to consider as indications for species-specific pesticide treatments during the storage period. If T. molitor is seen as a stored-product pest, a temperature that triggers management treatments is ~ 27.5 °C because it develops fast and would probably reproduce several times, issues that are linked with elevated infestations of stored commodities. In a recent study, Kavallieratos et al. (2021) showed that pirimiphos-methyl caused significantly higher mortalities to T. molitor larvae and adults when applied on barley, as temperature rose from 20 °C to 30 °C.
This study also revealed that T. molitor spends most of its lifetime at the stage of larva, followed by the stages of egg, and pupa. This is important because larvae of T. molitor are the tradable stage of this species for human consumption, animal feed, live fishing bait, and live/dried pet feed (Ng et al. 2001; Hill 2003; Bußler et al. 2016; Vandeweyer et al. 2017; Azzollini et al. 2018; Costa et al. 2020; Cho and Ryu 2021; Mazlum et al. 2021; Shafique et al. 2021; Shaviklo et al. 2021; Djouadi et al. 2022; Mouadi et al. 2022; Sriprablom et al. 2022). This is common phenomenon for stored-product insect species, even within the Tenebrionidae family, since the developmental duration of the larval stage prevails over immature stages. For example, O. surinamensis, T. castaneum, and T. confusum stay much longer as larvae than as eggs and pupae under different temperatures (Skourti et al. 2019; Nika et al. 2021; Kavallieratos et al. 2022) or T. granarium when growing on different grains such as barley, triticale, and oats (Kavallieratos et al. 2019).
Temperature impacts each stage separately. For instance, duration of T. molitor larvae reduces gradually from 22.5 °C to 25 °C, 27.5 °C, and 30 °C, which means that 22.5 °C are related to a delayed life cycle. Similar results were documented for O. surinamensis (20 °C – 32.5 °C), T. confusum (25 °C – 30 °C), and T. castaneum (20 °C – 30 °C) larvae (Skourti et al. 2019; Nika et al. 2021; Kavallieratos et al. 2022). Interestingly, after the increase of temperature from 32.5 to 35 °C, 30 to 32.5 °C, and 30 °C to 32.5 °C, developmental duration of O. surinamensis, T. confusum, and T. castaneum larvae decreased respectively (Skourti et al. 2019; Nika et al. 2021; Kavallieratos et al. 2022). The fact that temperature shortens the development of T. molitor can have an advantage towards multiple generations in a short time with a potential increased population of larvae. The acceleration of larval development at certain temperature levels fastens the emergence of pupae and adult individuals, thus the production of offspring (Skourti et al. 2019; Nika et al. 2021; Kavallieratos et al. 2022). Whether the short life cycle could suggest a potential reduction in the nutritional properties of the larvae for human and livestock consumption, needs further investigation. Even a small exposure to a different temperature can have impact on a certain developmental stage. When pupae of T. molitor were exposed to 4 °C for 15 days, the pupal period lasted ~ 5 days more than when pupae stayed only for 1 day at cold conditions (4 °C) (Sönmez and Koç 2019). Furthermore, as days of exposure to cold passed, the percentage of the emerged deformed adults reached ~ 76% after 15 days (Sönmez and Koç 2019). Whether specific temperatures cause morphometric alterations on T. molitor is yet to be unraveled. Our study revealed that the larval instars with the higher duration (i.e., the longest duration from one larval instar to another) were L16 to L26. The larval instars are separated by the observed exuviae (Park et al. 2014). By examining the instar periods and the body length of T. molitor when developed at 25 oC, Park et al. (2014) reported that both observed parameters were higher after the L16 instar than from L1 to L15. Since the more time a larva needs to successfully proceed to the next instar, the less exuviae will be generated during a specific time frame. Therefore, larvae from L16 to L26 are expected to produce lower number of exuviae than L1 to L15. This finding has several applications especially to the T. molitor trade due to its stay in food industries, pet shops, fish bait stores, and zoological parks (Ng et al. 2001; Vandeweyer et al. 2017). It has been previously documented that T. molitor causes rhinitis, contact erythema, itching, mild asthma, and rhinoconjunctivitis to humans (de Gier and Verhoeckx 2018; Beaumont et al. 2019; Nebbia et al. 2019; Ribeiro et al. 2021). Furthermore, it induces allergies to domestic animals such as dogs (Premrov-Bajuk et al. 2021). Exuviae of insects cause allergies to humans (Bernstein et al. 1983; Bellas 1990; Panzani and Ariano 2001; Yoder et al. 2007) as they can be classified as airborne contaminants (Ribeiro et al. 2021). It should be noted that T. molitor (e.g., whole larvae, exuviae, feces, excreta) are responsible for occupational asthma among fish bait handlers, grain mill workers, bakers, aquarists, and pet food workers (Bernstein et al. 1983; Siracusa et al. 2003; Rees 2004; Chan-Yeung et al. 2006; Gordon et al. 2006; Pacheco et al. 2013; de Gier and Verhoeckx 2018). Therefore, to reduce the hazard of exuviae in food and feed, larvae should be sifted with a sieve to be separated from the allergenic insect body parts, and consequently protect humans (Bernstein et al. 1983; Arévalo et al. 2022).
In conclusion, temperature has a crucial impact on the developmental duration of T. molitor when considering the confounders developmental stage and larval instar. On the basis of findings of the current study, rearing of this species between 22.5 °C and 30 °C is feasible. The lowest tested temperature of 22.5 °C favored the prolongation of the development of this species contrary to 25 °C, 27.5 °C, and 30 °C. From practical point of view, the selection of a certain temperature level tested in the current study will modify the developmental duration of T. molitor, an issue that will help industry to schedule the large-scale culture of this species. To moderate allergenic hazards, it is preferable to handle larvae from L16 to L26. Further research efforts are needed to shed light on more parameters (e.g., different strains) that affect the biology of T. molitor, due to its importance as food and feed.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RH:
-
Relative Humidity
- AIC:
-
Akaike Information Criterion
References
Adámková A, Adámek M, Mlček J, Borkovcová M, Bednářová M, Kouřimská L, Skácel J, Vítová E (2017) Welfare of the meal-worm (Tenebrio molitor) breeding with regard to nutrition value and food safety. Potravinarstvo Slovak J Food Sci 11:460–465. https://doi.org/10.5219/779
Adámková A, Mlček J, Adámek M, Borkovcová M, Bednářová M, Hlobilová V, Knížková I, Juríková T (2020) Tenebrio molitor (Coleoptera: Tenebrionidae)–Optimization of rearing conditions to obtain desired nutritional values. J Insect Sci 20:24. https://doi.org/10.1093/jisesa/ieaa100
Adamski Z, Bufo SA, Chowański S, Falabella P, Lubawy J, Marciniak P, Pacholska-Bogalska J, Salvia R, Scrano L, Słocińska M, Spochacz M, Szymczak M, Urbański A, Wałkowiak-Nowicka K, Rosiński G (2019) Beetles as model organisms in physiological, biomedical and environmental sciences - a review. Front Physiol 10:319. https://doi.org/10.3389/fphys.2019.00319
Arévalo HAA, Rojas EMM, Fonseca KBB, Mejía SMV (2022) Implementation of the HACCP system for production of Tenebrio molitor larvae meal. Food Control 138:109030. https://doi.org/10.1016/j.foodcont.2022.109030
Athanassiou CG, Kavallieratos NG, Campbell JF (2017) Competition of three species of Sitophilus on rice and maize. PLoS ONE 12:e0173377. https://doi.org/10.1371/journal.pone.0173377
Athanassiou CG, Kavallieratos NG, Throne JE, Nakas CT (2014) Competition among species of stored-product psocids (Psocoptera) in stored grain. PLoS ONE 9:e102867. https://doi.org/10.1371/journal.pone.0102867
Azzollini D, Derossi A, Fogliano V, Lakemond CMM, Severini C (2018) Effects of formulation and process conditions on micro-structure, texture and digestibility of extruded insect-riched snacks. Innov Food Sci Emerg Technol 45:344–353. https://doi.org/10.1016/j.ifset.2017.11.017
Beaumont P, Courtois J, Van der Brempt X, Tollenaere S (2019) Food-induced anaphylaxis to Tenebrio molitor and allergens implicated. Rev Fr Allergol 59:389–393. https://doi.org/10.1016/j.reval.2019.06.001
Bellas TE (1990) Occupational inhalant allergy to arthropods. Clin Rev Allergy 8:15–29. https://doi.org/10.1007/BF02914434
Bernstein DI, Gallagher JS, Bernstein IL (1983) Mealworm asthma: clinical and immunologic studies. J Allergy Clin Immunol 72:475–480. https://doi.org/10.1016/0091-6749(83)90584-5
Bjørge JD, Overgaard J, Malte H, Gianotten N, Heckmann LH (2018) Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J Insect Physiol 107:89–96. https://doi.org/10.1016/j.jinsphys.2018.02.010
Bußler S, Rumpold BA, Jander E, Rawel HM, Schlüter OK (2016) Recovery and technofunctionality of flours and proteins from two edible insect species: mealworm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2:e00218. https://doi.org/10.1016/j.heliyon.2016.e00218
Chan-Yeung M, Bernstein IL, Von Essen S, Singh J, Schwartz DA (2006) Acute Airway Diseases Due to Organic Dust Exposure. In: Bernstein IL, Chan-Yeng M, Malo JL, Bernstein DI (eds) Asthma in the workplace. Taylor and Francis Group, New York, pp 641–682
Chen F, Jiang H, Gan Y, Chen W, Huang G (2019) Optimization of hydrolysis conditions for obtaining antithrombotic peptides from Tenebrio molitor larvae. Am J Biochem Biotechnol 15:52–60. https://doi.org/10.3844/ajbbsp.2019.52.60
Cho SY, Ryu GH (2021) Effects of mealworm larva composition and selected process parameters on the physicochemical properties of extruded meat analog. Food Sci Nut 9:4408–4419. https://doi.org/10.1002/fsn3.2414
Costa S, Pedro S, Lourenço H, Batista I, Teixeira B, Bandarra NM, Murta D, Nunes R, Pires C (2020) Evaluation of Tenebrio molitor larvae as an alternative food source. NFS J 21:57–64. https://doi.org/10.1016/j.nfs.2020.10.001
Deruytter D, Coudron CL, Claeys J (2022) The effects of density on the growth and temperature production of Tenebrio molitor larvae. Sustainability 14:6234. https://doi.org/10.3390/su14106234
De Souza PC, Morey AT, Castanheira GM, Bocate KP, Panagio LA, Ito FA, Furlaneto MC, Yamada-Ogatta SF, Costa IN, Mora-Montes HM, Almeida RS (2015) Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J Microbiol Methods 118:182–186. https://doi.org/10.1016/j.mimet.2015.10.004
Ding Q, Wu RA, Shi T, Yu Y, Yan Y, Sun N, Sheikh AR, Luo L, He R, Ma H (2021) Antiproliferative effects of mealworm larvae (Tenebrio molitor) aqueous extract on human colorectal adenocarcinoma (Caco-2) and hepatocellular carcinoma (HepG2) cancer cell lines. Food Biochem 45:e13778. https://doi.org/10.1111/jfbc.13778
Djouadi A, Sales JR, Carvalho MO, Raymundo A (2022) Development of healthy protein-rich crackers using Tenebrio molitor flour. Foods 11:702. https://doi.org/10.3390/foods11050702
Eberle S, Schaden LM, Tintner J, Stauffer C, Schebeck M (2022) Effect of temperature and photoperiod on development, survival, and growth rate of mealworms Tenebrio molitor. Insects 13:321. https://doi.org/10.3390/insects13040321
Espinoza-Pinchi J, Ordonez-Galvez J, Castaneda-Olivera CA, Benites Alfaro EG (2022) Environmental biotechnology: biodegradation of microplastics with larvae of Tenebrio molitor and Galleria mellonella. Chem Eng Trans 93:187–192. https://doi.org/10.3303/CET2293032
de Gier S, Verhoeckx K (2018) Insect (food) allergy and allergens. Mol Immunol 100:82–106. https://doi.org/10.1016/j.molimm.2018.03.015
Gordon S, Bush RK, Newman-Taylor AJ (2006) Laboratory Animal, Insect, Fish, and Shellfish Allergy. In: Bernstein IL, Chan-Yeng M, Malo JL, Bernstein DI (eds) Asthma in the workplace. Taylor and Francis Group, New York, pp 415–436
Greene WH (2003) Econometric Analysis. Prentice-Hall, New Jersey
Hagstrum DW, Subramanyam B (2009) Stored-product insect resource. AACC Inc, St Paul
Hill DS (2003) Pests of storage foodstuffs and their control. Kluwer Academic Publishers, New York
Honěk A (1996) Life history and development. In: Hodek I, Honěk A (eds) Ecology of Coccinellidae. Kluwer Academic Publishers, Dordrecht, pp 61–93
Hwang D, Lee SH, Goo TW, Yun EY (2022) Potential of antimicrobial peptide-overexpressed Tenebrio molitor larvae extract as a natural preservative for Korean traditional sauces. Insects 13:381. https://doi.org/10.3390/insects13040381
Kavallieratos NG, Athanassiou CG, Guedes RNC, Drempela JD, Boukouvala MC (2017) Invader competition with local competitors: displacement or coexistence among the invasive khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae), and two other major stored-grain beetles? Front Plant Sci 8:1837. https://doi.org/10.3389/fpls.2017.01837
Kavallieratos NG, Eleftheriadou N, Boukouvala MC, Skourti A, Filintas CS, Gidari DLS, Maggi F, Rossi P, Drenaggi E, Morshedloo MR, Ferrati M, Spinozzi E (2024) Exploring the efficacy of four Apiaceae essential oils against nine stored-product pests in wheat protection. Plants 13:533. https://doi.org/10.3390/plants13040533
Kavallieratos NG, Karagianni ES, Papanikolaou NE (2019) Life history of evaluation of Trogoderma granarium Everts (Coleoptera: Dermestidae) on peeled barley, peeled oats and triticale. J Stored Prod Res 84:101515. https://doi.org/10.1016/j.jspr.2019.101515
Kavallieratos NG, Nika EP, Pražić-Golić M, Adrić G, Skourti A, Papanikolaou NE (2022) Impact of temperature on life history of two long-term laboratory strains of Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) from Greece and Serbia. J Stored Prod Res 96:101937. https://doi.org/10.1016/j.jspr.2022.101937
Kavallieratos NG, Papanikolaou NE, Kazani AN, Boukouvala MC, Malesios C (2021) Using multilevel models to explore the impact of abiotic and biotic conditions on the efficacy of pirimiphos-methyl against Tenebrio molitor L. Environ Sci Pollut Res 28:17200–17207. https://doi.org/10.1007/s11356-020-11925-3
Klost M, Ramirez-Huerta MI, Drusch S (2022) Heat-induced gelation of protein from mealworm (Tenebrio molitor): influence of pH and zinc concentration. Food Hydrocoll Health 2:100105. https://doi.org/10.1016/j.fhfh.2022.100105
Lozoya-Pérez NE, García-Carnero LC, Martínez-Álvarez JA, Martínez-Duncer I, Mora-Montes HM (2021) Tenebrio molitor as an alternative model to analyze the Sporothrix species virulence. Infect Drug Resist 14:2059–2072. https://doi.org/10.2147/IDR.S312553
Mazlum Y, Turan F, Yildirim YB (2021) Evaluation of mealworms (Tenebrio molitor) meal as an alternative protein source for narrow-clawed crayfish (Pontastacus leptodactylus) juveniles. Aquat Res 52:4145–4153. https://doi.org/10.1111/are.15253
Mouadi J, Pafilis P, Elbahi A, Okba Z, Elouizgani H, Mouden EHE, Aourir M (2022) The effect of weight and prey species on gut passage time in an endemic gecko Quedenfeldtia moerens (Chabanaud, 1916) from Morocco. Acta Herpetol 17:21–26. https://doi.org/10.36253/a_h-12326
Nebbia S, Lamberti C, Giorgis V, Giuffrida MG, Manfredi M, Marengo E, Pessione E, Schiavone A, Boita M, Brussino L, Cavallarin L, Rolla G (2019) The cockroach allergen-like protein is involved in primary respiratory and food allergy to yellow mealworm (Tenebrio molitor). Clin Exp Allergy 49:1379–1382. https://doi.org/10.1111/cea.13461
Ng WK, Liew FL, Ang LP, Wong KW (2001) Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquac Res 32:273–280. https://doi.org/10.1046/j.1355-557x.2001.00024.x
Nika EP, Kavallieratos NG, Papanikolaou NE, Malesios C (2022a) Interactions of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) with two key stored-product pests under variable abiotic conditions. Entomol Gen 42:471–478. https://doi.org/10.1127/entomologia/2022/1438
Nika EP, Kavallieratos NG, Malesios C (2022b) Dangerous liaisons of three key secondary stored-product pests in cracked maize. J Stored Prod Res 99:102037. https://doi.org/10.1016/j.jspr.2022.102037
Nika EP, Kavallieratos NG, Papanikolaou NE (2021) Linear and non-linear models to explain influence of temperature on life history traits of Oryzaephilus surinamensis (L.). Entomol Gen 41:157–167. https://doi.org/10.1127/entomologia/2020/1088
Pacheco KA, Gautrin D, Lopata AL, Jeebhay MF (2013) Asthma and Allergy to Animals. In: Malo JL, Chan-Yeung M, Bernstein DI (eds) Asthma in the workplace. CRC Press, Boca Raton, pp 238–261
Panzani RC, Ariano R (2001) Arthropods and invertebrates allergy (with the exclusion of mites): the concept of panallergy. Allergy 56:1–22. https://doi.org/10.1111/j.1398-9995.2001.tb04419.x
Papanikolaou NE, Kavallieratos NG, Boukouvala MC, Malesios C (2018) Do temperature, relative humidity and interspecific competition alter the population size and the damage potential of stored-product insect pests? A hierarchical multilevel modeling approach. J Therm Biol 78:415–422. https://doi.org/10.1016/j.jtherbio.2018.10.022
Papanikolaou NE, Kavallieratos NG, Kondakis M, Boukouvala MC, Nika EP, Demiris N (2019) Elucidating fitness components of the invasive dermestid beetle Trogoderma granarium combining deterministic and stochastic demography. PLoS ONE 14:e0212182. https://doi.org/10.1371/journal.pone.0212182
Park JB, Choi WH, Kim SH, Jin HJ, Han YS, Lee YS, Kim NJ (2014) Developmental characteristics of Tenebrio molitor larvae (Coleoptera: Tenebrionidae) in different instars. Int J Indust Entomol 28:5–9. https://doi.org/10.7852/ijie.2014.28.1.5
Peng BY, Chen Z, Chen J, Zhou X, Wu WM, Zhang Y (2021) Biodegradation of polylactic acid by yellow mealworms (larvae of Tenebrio molitor) via resource recovery: a sustainable approach for waste management. J Hazard Mater 416:125803. https://doi.org/10.1016/j.jhazmat.2021.125803
Premrov-Bajuk B, Zrimšek P, Kotnik T, Leonardi A, Križaj I, Jakovac-Strajn B (2021) Insect protein-based diet as potential risk of allergy in dogs. Animals 11:1942. https://doi.org/10.3390/ani11071942
Rees D (2004) Insects of stored products. Manson Publishing, London
Ribeiro JC, Sousa-Pinto B, Fonseca J, Caldas-Fonseca S, Cunha LM (2021) Edible insects and food safety: allergy. J Insects Food Feed 7:833–847. https://doi.org/10.3920/JIFF2020.0065
Robinson WH (2005) Urban insects and arachnids. Cambridge University Press, Cambridge
Shafique L, Abdel-Latif HMR, Hassan FU, Alagawany M, Naiel MAE, Dawood MAO, Yilmaz S, Liu Q (2021) The feasibility of using yellow mealworms (Tenebrio molitor): towards a sustainable aquafeed industry. Animals 11:811. https://doi.org/10.3390/ani11030811
Shaviklo AR, Alizadeh-Ghamsari AH, Hosseini SA (2021) Sensory attributes and meat quality of broiler chickens fed with mealworm (Tenebrio molitor). J Food Sci Technol 58:4587–4597. https://doi.org/10.1007/s13197-020-04946-w
Siracusa A, Marcucci F, Spinozzi F, Marabini A, Pettinari L, Pace ML, Tacconi C (2003) Prevalence of occupational allergy due to live fish bait. Clin Exp Allergy 33:507–510. https://doi.org/10.1046/j.1365-2222.2003.01641.x
Skourti A, Kavallieratos NG, Papanikolaou NE (2019) Laboratory evaluation of development and survival of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) under constant temperatures. J Stored Prod Res 83:305–310. https://doi.org/10.1016/j.jspr.2019.07.009
Song DH, Kim M, Jin ES, Sim DW, Won HS, Kim EK, Jang S, Choi YS, Chung KH, An JH (2019) Cryoprotective effect of an antifreeze protein purified from Tenebrio molitor larvae on vegetable. Food Hydrocoll 94:585–591. https://doi.org/10.1016/j.foodhyd.2019.04.007
Sönmez E, Koç Y (2019) Effects of cold exposure on Tenebrio molitor (Coleoptera: Tenebrionidae) pupal period, proportion of adult emergence, weight and deformation percentage. Entomol Fennica 30:43–48. https://doi.org/10.33338/ef.79905
Sriprablom J, Kitthawee S, Suphantharika M (2022) Functional and physicochemical properties of cookies enriched with edible insect (Tenebrio molitor and Zophobas atratus) powders. Food Meas Charact 16:2181–2190. https://doi.org/10.1007/s11694-022-01324-2
Sugumar P, Sha DSM, Gowda S, Vijay T, Keerthana S (2022) An assessment on the potential of Tenebrio molitor used for biodepolymerization of plastics and polystyrene: influencing factors, various feeding cases and gut microbiota. IOP Conf Ser: Earth Environ Sci 1074:012029. https://doi.org/10.1088/1755-1315/1074/1/012029
The R Project for Statistical Computing (2023) Available online: http://www.r-project.org. Accessed 28 Feb 2023
Vandeweyer D, Crauwels S, Lievens B, Van Campenhout L (2017) Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllo dessigillatus) from different rearing companies and different production batches. Int J Food Microbiol 242:13–18. https://doi.org/10.1016/j.ijfoodmicro.2016.11.007
Vigneron A, Jehan C, Rigaud T, Morel Y (2019) Immune defenses of a beneficial pest: the mealworm beetle, Tenebrio molitor. Front Physiol 10:138. https://doi.org/10.3389/fphys.2019.00138
Vommaro ML, Kurtz J, Giglio A (2021) Morphological characterization of haemocytes in the mealworm beetle Tenebrio molitor (Coleoptera: Tenebrionidae). Insects 12:423. https://doi.org/10.3390/insects12050423
Yang SS, Brandon AM, Flanagan JCA, Yang J, Ning D, Cai SY, Fan HQ, Wang ZY, Ren J, Benbow E, Ren NQ, Waymouth RM, Zhou J, Criddle CS, Wu WM (2018) Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere 191:979–989. https://doi.org/10.1016/j.chemosphere.2017.10.117
Yang SS, Ding MQ, Zhang ZR, Ding J, Bai SW, Cao GL, Zhao L, Pang JW, Xing DF, Ren NQ, Wu WM (2021) Confirmation of biodegradation of low-density polyethylene in dark- versus yellow- mealworms (larvae of Tenebrio obscurus versus Tenebrio molitor) via. gut microbe-independent depolymerization. Sci Total Environ 789:147915
Yoder JA, Glenn BD, Benoit JB, Zettler LW (2007) The giant Madagascar hissing-cockroach (Gromphadorhina portentosa) as a source of antagonistic moulds: concerns arising from its use in a public setting. Mycoses 51:93–98. https://doi.org/10.1111/j.1439-0507.2007.01470.x
Funding
Open access funding provided by HEAL-Link Greece. The study is partially funded by the project 34.0889 (Special Account for Research Funds, Agricultural University of Athens, Greece).
Author information
Authors and Affiliations
Contributions
NGK: Conceptualization, data curation, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing, supervision. AS: data curation, investigation, writing—original draft, writing—review and editing. EPN: Data curation, investigation, writing—original draft, writing—review and editing. CM: Data curation, formal analysis, software, investigation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that there have no conflicts of interest.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kavallieratos, N.G., Skourti, A., Nika, E.P. et al. Development of the edible Tenebrio molitor at different temperatures: a Poisson log-linear regression modeling approach. Biologia 79, 2059–2069 (2024). https://doi.org/10.1007/s11756-024-01675-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01675-9