Abstract
Barley straw has been used around the world for decades as a simple, cost-effective and harmless phytoplankton growth inhibition method. Although the effect of this method on algal blooms is quite well depicted, it has not yet been tested what effect it has on submerged vascular plants and if it could be used to control the spread of alien macrophytes. In this study two highly invasive plant species: Cabomba caroliniana and Elodea nuttallii were exposed to different concentration of the barley straw extract (BSE) in laboratory conditions for a duration of four weeks. In the course of the investigations, responses of 8 traits associated with growth, biomass and chlorophyll concentration of specimens to three dosages (Low, Medium, High) of BSE corresponding to concentrations: 0.03, 0.30 and 1.50 ml l−1 were analysed. The result showed that although dry mass and total length of the plants did not differ significantly between the test groups, increase in tillering and internodes number was observed for certain concentrations of the extract. This shows that if BSE has any effect on submerged macrophytes it is a positive one and thus the method is not suitable for invasive submerged aquatic plant control. Furthermore, it is recommended that before using barley straw for algae bloom control one should make sure that there are no alien aquatic submerged plants in the area that could benefit from such a treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past century there has been a sharp decline in the biodiversity around the world (Cardinale et al. 2012; Naggs 2017). Invasive alien species are believed to be one of the major causes for the biodiversity loss (Cafaro 2015). Their rapid expansion leads not only to serious disruption along global ecosystems but also causes enormous economic losses to many economies around the world. It is estimated that alien species generate losses in tens of billions of Euro for European Union (Kettunen et al. 2009) and more than one hundred billion dollars for United States of America (Pimentel et al. 2005) annually alone. However these sums are likely greatly underestimated since the existing cost reports describe only a fracture of actual costs and that is just for only a small number of alien species (Haubrock et al. 2021). Furthermore we can expect that in future the problem of biological invasions will only grow in magnitude (Seebens et al. 2017). Thus taking everything into an account it is not surprising that the alien invasions and effective methods of their management are getting more and more attention.
Almost 10% of global biodiversity is associated with freshwater ecosystems despite the fact that such habitats cover less than 1% of Earth’s surface (Dudgeon 2019). It is therefore extremely alarming that these biodiversity hotspots are threatened even more than majority of terrestrial ecosystems (Dudgeon et al. 2006). Aquatic plants are well known for the complex impact they have on aquatic environment and thus it is not surprising that mass development of invasive aquatic plants may violently disrupt the functioning of the entire ecosystem (Tasker et al. 2022). Unfortunately management and eradication of alien aquatic species is considered to be an exceptionally difficult task (Simberloff 2021) since any management technique is more difficult to perform under the water surface. Additionally many commonly used methods for invasive aquatic plant control have serious drawbacks which in case of certain species can make them less effective or even counterproductive. For example, various mechanical and physical techniques are widely used to control mass development of invasive macrophytes (Hussner et al. 2017). However, such methods are usually not only not species specific but may also produce great number of plant fragments which in case of many invasive plant species can then regrow rapidly or even be easily transported to new sites. Due to the characteristics of freshwater ecosystems also herbicide-using techniques potentially pose a great risk to non-target organisms (Simberloff 2021) and their use is forbidden in many countries. In view of the limitations of current methods, and ever-increasing pressure from invasive macrophytes, the search for new control techniques seems to be extremely important.
Barley straw has been widely used for decades in order to inhibit algae blooms in nutrient polluted waterbodies (Newman and Barrett 1993; Barrett et al. 1999; Ó hUallacháin et al. 2010; Rajabi et al. 2010; Islami and Filizadeh 2011). Popularity of this method results from its cheapness, simplicity and perhaps most importantly, its alleged environmental-friendliness (Newman 2012; Pęczuła and Suchora 2014; Fervier et al. 2020). Despite the fact that the method has been in use for more than 30 years, the exact mechanism standing behind the barley’s effects was not fully understood for the majority of time. It was believed, however that lignin derivatives were involved. More recent researches (Xiao et al. 2014) indicated that decomposing barley straw releases into the environment two substances belonging to the group of polyphenolics: Salcolin A and Salcolin B which effectively inhibit algae growth (Zhu et al. 2021). However, barley straw is also known to produce great range of almost 50 other allelochemicals which make this plant widely used also for weed control in agriculture (Kremer and Ben-Hammouda 2009; Arias and Bhatia 2015). Although the topic of the effect of barley straw on algae blooms is fairly well studied, its potential effect on aquatic vascular plants has not yet been studied well. Up to this point only Pęczuła and Suchora (2014) tested its direct effect on a single species of aquatic vascular plant: freefloating duckweed (Lemna valdiviana Phil.). They found that small doses of barley straw could slow down the growth of this alien for Europe species of duckweed. However, Fervier et al. (2020) in their work examining the effects of various control methods on the phytoplankton noticed significant increase in growth and biomass of Stuckenia pectinata (L.) Börner and Myriophyllum spicatum L. in trials treated with barley straw. The effect of barley straw extract (BSE) on plants is more well described for the terrestrial plants (Ben-Hammouda et al. 2001; Chon and Kim 2004; Dhima et al. 2008). Its strong negative effects on plant growth and development have led to its use as an herbicide in agriculture for years.
Barley straw and its extracts are often used to combat algae blooms in eutrophic waterbodies that often are also under strong anthropogenic pressure. Such disturbed habitats are very vulnerable to aquatic invasions and often succumb to them. It is thus interesting to find out if barley straw has any impact on the growth and development of alien aquatic plants. If negative impact would be demonstrated we could get a new, cheap and environmental friendly tool in our effort to control the spread of invasive aquatic species. However positive reaction of alien macrophytes would mean that more caution would be advised while applying this method to habitats exposed to aquatic plant invasion.
The impact of restoration of various water bodies, especially lakes using a method based on BSE, is still poorly understood in terms of its effect on macrophytes. Moreover, despite studies on the effects of BSE on macrophytes (Pęczuła and Suchora 2014), no specific mechanism to explain its toxicity can be said to have been identified. In order to fill this gap, a laboratory experiment was developed to study the effect of BSE on the growth of two species Elodea nuttallii and Cabomba caroliniana. It was assumed that the application of BSE would cause deterioration of abiotic conditions with an intensity proportional to the dose. Consequently, energy will be diverted to survive adverse changes, resulting in growth inhibition. Accordingly, the purpose of this work is to test if barley straw may have any effect on the growth of alien macrophytes and thus if it could it be used as an easy and cheap alternative to other suppression techniques.
Materials and methods
Choice of species and barley straw extract
In order to test the impact of BSE on alien aquatic plant we chose two species: Elodea nuttallii (Planch.) H. St. John (Nuttall’s waterweed) and Cabomba caroliniana A. Gray (Carolina fanwort). Both species are considered to be highly invasive and their rapid spread is observed in both Europe and Asia. Cabomba caroliniana and Elodea nuttallii colonize both natural and heavily human-altered waters often characterized by high trophic levels (Greulich and Tremolieres 2006; Matthews et al. 2013). Their ability to rapid grow and their large impact on native ecosystems resulted in their inclusion on the List of invasive alien species of Union concern EU (Commission Implementing Regulation (Eu) 2016/1141). Since these species are difficult to eradicate, it is essential to take appropriate management and control measures.
Plants as well as the water used in the experiment were collected at the early October from two different locations in Poland as these two plant species do not occur together in this country. E. nuttallii was gathered from lake Skoki (Kujawsko-Pomorskie voivodeship, Poland; N 52°36’22.49”, E 19°23’39.43”) while C. caroliniana was collected from its sole known location in Poland: an artificial pond in the village Krążek (Małopolska voivodeship, Poland; N 50°17’26.75”, E 19°27’11.99”). Commercially available barley straw (Hordeum vulgare L.) extracts are widely popular and used worldwide to combat algae blooms. Due to the ease of application and immediate effect it is often used in place of bales of barley straw, especially in personal use. For this study MICROBE-LIFT Barley Straw Concentrated Extract from USA company Ecological Laboratories was chosen. This product is one of the most popular BSE available on the market and its effect has been demonstrated in the environment (Gonzalez Rueda 2009).
Experimental design
In order to test the effect of BSE on aquatic plants, 150 healthy and similar-looking top shoots were collected for each species. In the next step plants were cleaned and cut in such manner that each shoot had the same length (11 cm for E. nuttallii and 13 cm for C. caroliniana). Subsequently, each individual was placed in a tank filled with filtered water collected from the same location as the plants. The specimens were then left for a whole week to acclimatize. After that 126 similar and healthy-looking plants were collected for each species. They were then cut to the same length as before acclimatization. In the next step, the following characteristics were measured for each individual: number of internodes for main shoot and offshoots, length and number of offshoots. Additionally, 30 individuals were then randomly selected for each species for chlorophyll a measurement after which the plants were dried do determine their dry mass. Remaining plants of each species were then randomly divided into four groups of 24 individuals each. The first group was the control group, second was to be exposed to the recommended by producer dose of the BSE (Low, 0.03 ml/L), the third to ten times the dose (Medium, 0.30 ml/L) and the last to fifty times the dose (High, 1.50 ml/L).
The used doses are similar to those applied in other studies that used MICROBE-LIFT Barley Straw Concentrated Extract (Schrader 2005; Gonzalez Rueda 2009; Lürling et al. 2016) which ranged in general from 0.005 to 15 ml/L. Doses were selected with the assumption that even the lowest dose should be sufficient to reduce phytoplankton biomass (Gonzalez Rueda 2009). The higher doses were a multiplication of the lowest dose, although all doses could be classified as used in lakes and fish ponds revitalization. Plants from each group were then transferred to one of the six tanks, resulting in a final total of four individuals for each tank and thus six replications for each dosage per species. The vessels were then filled with filtered through a GF/C fiber glass filter water which was gathered from the same site that the plants were collected. Finally, the appropriate amount of BSE for the each group was added. For E. nuttallii tanks with volume of 2 L (glass cylinders, height of water column 19 cm) were used while tanks used for C. caroliniana had volume of 3 L (glass cylinders, height of water column 23 cm). The plants were then kept for four weeks under stable conditions with a constant temperature of 20 degrees, a 12-hour day/night cycle and with a light intensity of 160,6 µmol m−2 s−1. During this time, additional doses of BSE were added once per week, accordingly to the producer’s recommendation. These doses were equal to a quarter of the initial doses (accordingly: control, 0.015 ml/L, 0.15 ml/L and 0.75 ml/L). Following the end of the first week water temperature, pH, electric conductivity, dissolved oxygen, oxygen saturation and TDS (ProDSS Multiparameter Digital Water Quality Meter, YSI) were measured twice a week for each sample. The summary of the water chemistry data for the research experiment treatment is presented Table 1.
After four weeks, all plants were taken out of the tanks and measured once again. This time chlorophyll a content was also measured for every specimen. For all chlorophyll measurements CCM-300 Chlorophyll Content Meter was used. Finally, the specimens were dried to determine their dry weight. For all statistical analyses R program (R Core Team 2023) was used. One-way analysis of variance (ANOVA) followed by Tukey’s test were performed to determine differences between test groups. All figures were also prepared with R program using the ggplot2 package.
Results
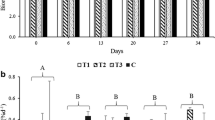
After the addition BSE no visible differences in color or turbidity of water were observed between tanks. Water electric conductivity, TDS and pH has not changed significantly over the course of the study (Table 1). Throughout the whole study period, oxygen saturation and dissolved oxygen levels for D1, D10 and D50 doses were high and typically higher than in the control (Figs. 1, 2, 3 and 4) for both of species. The decrease in dissolved oxygen was observed only for Cabomba caroliniana after a week of exposure to the highest dose of BSE. However, after another 48 h, oxygen levels equalized with the rest of treatments. Overall, oxygen saturation levels throughout the experiment were lower for E. nuttallii compared to C. caroliniana (Figs. 3 and 4).
All individuals reached the end of the experiment alive and seemingly in good condition. The length of the main shoot for both C. caroliniana and E. nuttallii differed significantly depending on the concentration of BSE (Tables 2 and 3, Online Resource 1 in the supplementary material). However, the response was different for both species. In case of C. caroliniana the longest shoots were observed for the highest concentrations of the extract (Fig. 1a). The situation was the opposite for the E. nuttallii (Fig. 2a) whereas the concentration increased the length of the main shoot decreased. For the summed lengths of the main and lateral shoots no significant differences between the doses for both species were found. However, for C. caroliniana once again the highest mean value was observed for plants exposed to the highest concentration of the extract (Fig. 1b). For E. nuttallii the highest mean value of summed main and lateral shoots length (Fig. 2b) was observed for plants grown at the producer’s recommended concentration of BSE – Low dose (0,03 ml/L). A significant change in offshoot number was noted only for C. caroliniana (Fig. 1c). In the case of this species, increased plant tillering was observed but only for individuals exposed to the highest dose of the extract. The mean length of lateral shoots and the total sum of their length appeared to be unaffected by different doses of the BSE (Table 2). However, again in the case of C. caroliniana total lateral shoot length was highest for the individuals exposed to the highest concentration of barley straw. The total number of internodes differed significantly between groups for both species (Figs. 1d and 2d). For E. nuttallii individuals exposed to minimal and medium doses of extract produced the most internodes while for C. caroliniana the highest number of internodes was observed for individuals exposed to the maximum dose of BSE. No flowering specimens were found for both of the species. Significant differences and changes were also observed for the amount of chlorophyll a (Figs. 1f and 2f). Under laboratory conditions its content increased compared to its pre experiment value for C. caroliniana and decreased for E. nuttallii. However, for both species the highest chlorophyll values were observed for individuals from control group or in the case of E. nuttallii those also exposed only to the minimal dose of barley extract. Despite all the above differences in plant morphology, plant dry weight appeared to be similar between all testing groups and was not statistically significantly different for either of species (Figs. 1e and 2e). Nevertheless, it was once again the case that the highest mean value of dry mass for C. caroliniana was observed for individuals exposed to the maximum extract concentration. In general, C. caroliniana seemed to be most positively stimulated by the highest doses of BSE (High, 1.50 ml/L) while E. nuttallii usually reached the highest average values for the lowest dose (Low, 0.03 ml/L).
Discussion
Both Cabomba caroliniana and Elodea nuttallii are considered difficult to control and eradicate (Zehnsdorf et al. 2015; Roberts and Florentine 2022) aquatic plant species. Both are known for the strong impact they have on biodiversity and ecosystem services (Nagasaka et al. 2002; Hogsden et al. 2007). In this work, we showed how two widely dispersed aquatic invasive species responded to the use of alternative to chemical and mechanical methods of control. Nowadays, an alternative to chemical methods is being sought due to concerns about their high environmental harm, and the ever-increasing resistance of plants to synthetic herbicides (de Souza Barros et al. 2021). Biological herbicides in the form of plant derived extracts are much more environmentally friendly, while remaining inexpensive and simple to apply. Few studies dealing with this topic shows that extracts derived from certain plants such as Artemisia dracunculus L., Artemisia vulgaris L. (Oduro et al. 2005), Parthenium hysterophorus L. (Pandey 1994) and Hordeum vulgare (Pęczuła and Suchora 2014) may successfully limit the growth and development of aquatic alien plant species.
Although the results showed some effect of BSE on the morphology of invasive aquatic plants, no strong negative effect on the growth rate of individuals was observed. Thus, the negative effect of barley straw on biomass growth that was demonstrated for Lemna valdiviana (Pęczuła and Suchora 2014) was not observed for submerged vascular plants. While there was no statistically significant negative or positive effect on biomass between groups for any of the species, a positive effect on plant tillering was observed for certain values of extract concentration. In the case of C. caroliniana, exposure to the highest concentration of the extract (High, 1.50 ml/L) resulted with the individuals featuring highest number of internodes and offshoots. Mean dry mass, although not statistically different from that of the control group was also highest for this concentration of barley straw. For E. nuttallii, individuals with the highest total length, number of internodes and longest lateral shoots were observed when producer’s recommended dose was applied (Low, 0.03 mg l/L). These results show that if BSE has any effect on submerged alien aquatic plants it is rather positive one and therefore this substance should not be used as a method of their control.
Few studies undertake an evaluation of combine effect that BSE and submerged macrophytes have on algae. In their work in which Fervier et al. (2020) tested the effectiveness of the BSE in the presence of macrophytes, it is concluded that the main effect of the BSE application is promotion of aquatic plants growth. This, in turn, is supposed to allow macrophytes to compete with algae more effectively and has the indirect effect of plankton blooms inhibition. However, microcosm experiments in culture media with fishpond water demonstrated that macrophytes and BSE while used separately exhibit comparable negative effect on phytoplankton growth (Ghobrial et al. 2007). Under aerobic conditions that result from macrophyte activity, BSE causes significant reduction of phosphorus and other nutrients in water (Ghobrial et al. 2007). Such changes in the availability of essential nutrients should affect the growth and development of the plants as such. However, in our study, dissolved oxygen content in water, which reflects plant photosynthetic activity and their growth, was not correlated with the amount of BSE doses.
Three types of plant responses were recorded in response to increasing doses of BSE: (1) increase in the growth of the main shoot, (2) increase in the offshoot growth rate and (3) reduction of chlorophyll a content in shoots and leaves. Such plant behavior does not correspond to any of the life strategies described so far for aquatic plant species under stressful conditions. The application of BSE did not cause a change in the color and turbidity of the water thus the observed changes in plant morphology cannot be explained as a response to the disruption in light availability. As a result of intensive growth in length and lateral shoots, the assimilative area of macrophytes was multiplied. This was accompanied however, by a decrease of chlorophyll a content in shoots and leaves. In summary, the results seem to confirm that barley straw itself is safe for submerged vascular plants and its effect on such plants may even be positive.
There is a conclusion that may be drawn from the available literature and this study that the biological herbicides can have divergent effect on submerged plants and those free floating above the water surface. In all known cases the application of plant derived extracts shown to have negative effects on free floating plants such as: Lemna valdiviana (Pęczuła and Suchora 2014), Salvinia molesta D.S.Mitchell (Pandey 1994), Azolla filiculoides Lam. (Oduro et al. 2005) and a positive one on submerged plants such as: Stuckenia pectinata, Myriophyllum spicatum (Fervier et al. 2020), Cabomba caroliniana and Elodea nuttallii. Although further studies are required to confirm this observation, it can be assumed that biological herbicides such as BSE are especially suitable for the control of non-native free floating plant species. High specificity of such control method combined with its high environmental safety (Everall and Lees 1996; Boylan and Morris 2003) and the potential stimulation of submerged aquatic plants means that such substances could be used without concern for inflicting losses to the biodiversity of aquatic environments. Many of the most invasive aquatic macrophyte species are actually free floating plants. Their massive spread in lakes and rivers usually lead to the shading of large areas of these ecosystems. This in turn usually leads to changes in physical and chemical water conditions and results in a massive impoverishment of species richness. The use of natural herbicides such as BSE is therefore perhaps a way not only to limit their growth but also a method of boosting the health of the entire ecosystem through the stimulation of submerged macrophytes and algae growth limitation. Additional field studies are required to verify these conclusions. However if successful, the use of plant extracts would prove to be an excellent method in our fight to preserve the biodiversity of aquatic ecosystems all around the world.
On the contrary, caution is advised when plant extracts are being used for aquatic habitats that contain submerged invasive macrophytes. Although, aiding plant growth and tillering is usually a desirable outcome for native aquatic plant species, such an effect can have undesirable consequences in reservoirs under the pressure from submerged alien macrophytes. The success of invasive plant species is attributed in large part to their highly efficient asexual reproduction and very good regenerative abilities (Havel et al. 2015). Therefore, the increased number of internodes and offshoots means more fragments that can spread even further and colonize new sites. It should also be noted that in the natural environment, the positive effect of using barley straw on the growth of submerged invasive macrophytes is likely to be much more significant than the results of these studies might suggest. Barley straw is typically used for eutrophic freshwater ecosystems with turbid water and dominated by phytoplankton. The highly desirable goal of this method however, is not only a short-term inhibition of phytoplankton growth but permanent improvement in water clarity through a regime shift from phytoplankton to macrophyte dominance. The improved light conditions and the abundance of nutrients resulting from such a change is regrettably an opportunity not only for native but also invasive submerged aquatic plants (Caffrey and Monahan 1999). Such species most often do very well in such disturbed ecosystems and because of their high competitiveness they can spread rapidly and completely dominate local competition. Keeping this and the result of the experiment in mind, it is advisable to make sure that there are no alien submerged aquatic plant species in the area of treatment before applying barley straw as an algae or free floating aquatic plant growth suppressant.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Arias S, Bhatia SK (2015) Barley. In: Arias S, Bhatia SK (eds) Medical applications for biomaterials in Bolivia. SpringerBriefs in Public Health. Springer, Cham, pp 15–22. https://doi.org/10.1007/978-3-319-16775-6_2
Barrett PRF, Littlejohn JW, Curnow J (1999) Long-term algal control in a reservoir using barley straw. In: Caffrey J, Barrett PRF, Ferreira MT, Moreira IS, Murphy KJ, Wade PM (eds) Biology, ecology and management of aquatic plants. Developments in hydrobiology. Springer, Dordrecht, pp 309–313. https://doi.org/10.1007/978-94-017-0922-4_45
Ben-Hammouda M, Ghorbal H, Kremer R, Oueslati O (2001) Allelopathic effects of barley extracts on germination and seedlings growth of bread and durum wheats. Agronomie 21(1):65–71. https://doi.org/10.1051/agro:2001109
Boylan JD, Morris JE (2003) Limited effects of barley straw on algae and zooplankton in a midwestern pond. Lake Reserv Manag 19(3):265–271. https://doi.org/10.1080/07438140309354091
Cafaro P (2015) Three ways to think about the sixth mass extinction. Biol Conserv 192:387–393. https://doi.org/10.1016/j.biocon.2015.10.017
Caffrey JM, Monahan C (1999) Filamentous algal control using barley straw. Hydrobiologia 415:315–318. https://doi.org/10.1023/A:1003884211027
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486(7401):59–67. https://doi.org/10.1038/nature11148
Chon SU, Kim YM (2004) Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J Agron Crop Sci 190(2):145–150. https://doi.org/10.1111/j.1439-037X.2004.00088.x
de Souza Barros VM, Pedrosa JLF, Gonçalves DR, Medeiros FCLD, Carvalho GR, Gonçalves AH, Teixeira PVVQ (2021) Herbicides of biological origin: a review. J Hortic Sci Biotechnol 96(3):288–296. https://doi.org/10.1080/14620316.2020.1846465
Dhima K, Vasilakoglou I, Lithourgidis A, Mecolari E, Keco R, Agolli XH, Eleftherohorinos I (2008) Phytotoxicity of 10 winter barley varieties and their competitive ability against common poppy and ivy-leaved speedwell. Exp Agric 44(3):385–397. https://doi.org/10.1017/S001447970800639X
Dudgeon D (2019) Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr Biol 29(19):R960–R967. https://doi.org/10.1016/j.cub.2019.08.002
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81(2):163–182. https://doi.org/10.1017/S1464793105006950
Everall NC, Lees DR (1996) The use of barley-straw to control general and blue-green algal growth in a Derbyshire reservoir. Water Res 30(2):269–276. https://doi.org/10.1016/0043-1354(95)00192-1
Fervier V, Urrutia-Cordero P, Piano E, Bona F, Persson KM, Hansson LA (2020) Evaluating nutrient reduction, grazing and barley straw as measures against algal growth. Wetlands 40:193–202. https://doi.org/10.1007/s13157-019-01167-6
Ghobrial MG, Okbah MA, Gharib SM, Soliman AM (2007) Influence of barley straw and submerged macrophytes on fishpond wastewater quality. Egypt J Aquat Res 33(3):68–87
Gonzalez Rueda C (2009) Effets de la matière organique dissoute sur la croissance des espèces de cyanobactéries à la baie Missisquoi du lac Champlain. https://archipel.uqam.ca/2469/. Accessed 18 Mar 2023
Greulich S, Tremolieres M (2006) Present distribution of the genus Elodea in the Alsatian Upper Rhine floodplain (France) with a special focus on the expansion of Elodea nuttallii St. John during recent decades. Hydrobiologia 570:249–255. https://doi.org/10.1007/s10750-006-0188-y
Haubrock PJ, Turbelin AJ, Cuthbert RN, Novoa A, Taylor NG, Angulo E, Haubrock PJ, Turbelin AJ, Cuthbert RN, Novoa A, Taylor NG, Angulo E, Ballesteros-Mejia L, Bodey TW, Capinha C, Diagne C, Essl F, Golivets M, Kirichenko N, Kourantidou M, Leroy B, Renault D, Verbrugge L, Courchamp F (2021) Economic costs of invasive alien species across Europe. NeoBiota 67:153–190
Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB (2015) Aquatic invasive species: challenges for the future. Hydrobiologia 750:147–170. https://doi.org/10.1007/s10750-014-2166-0
Hogsden KL, Sager EP, Hutchinson TC (2007) The impacts of the non-native macrophyte Cabomba caroliniana on littoral biota of Kasshabog Lake, Ontario. J Great Lakes Res 33(2):497–504. https://doi.org/10.3394/0380-1330(2007)33[497:TIOTNM]2.0.CO;2
Hussner A, Stiers I, Verhofstad MJJM, Bakker ES, Grutters BMC, Haury J, Hussner A, Stiers I, Verhofstad MJJM, Bakker ES, Grutters BMC, Haury J, van Valkenburg JLCH, Brundu G, Newman J, Clayton JS, Anderson LWJ, Hofstra D (2017) Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat Bot 136:112–137. https://doi.org/10.1016/j.aquabot.2016.08.002
Islami HR, Filizadeh Y (2011) Use of barley straw to control nuisance freshwater algae. J Am Water Works Ass 103(5):111–118. https://doi.org/10.1002/j.1551-8833.2011.tb11458.x
Kettunen M, Genovesi P, Gollasch S et al (2009) Technical support to EU strategy on invasive species (IAS): assessment of the impacts of IAS in Europe and the EU (final module report for the European Commission). Brussels, Belgium. Institute for European Environmental Policy (IEEP), pp 43. https://ieep.eu/wpcontent/uploads/2009/11/ias_assessments.pdf. Accessed 4 Apr 2023
Kremer RJ, Ben-Hammouda M (2009) Allelopathic plants. 19. Barley (Hordeum vulgare L). Allelopathy J 24(2):225–241
Lürling M, Waajen G, de Senerpont Domis LN (2016) Evaluation of several end-of-pipe measures proposed to control cyanobacteria. Aquat Ecol 50:499–519. https://doi.org/10.1007/s10452-015-9563-y
Matthews J, Beringen R, Lamers LPM, Odé B, Pot R, Velde G et al (2013) Risk analysis of the non-native fanwort (Cabomba caroliniana) in the Netherlands. Reports Environmental Science 442. Department of Environmental Science, Institute for Water and Wetland Research, Radboud University Nijmegen, Nijmegen, The Netherlands. http://www.roelfpot.nl/publicaties/RA_Cabomba_2013.pdf. Accessed 4 Apr 2023
Nagasaka M, Yoshizawa K, Ariizumi K, Hirabayashi K (2002) Temporal changes and vertical distribution of macrophytes in Lake Kawaguchi. Limnology 3:107–114. https://doi.org/10.1007/s102010200012
Naggs F (2017) Saving living diversity in the face of the unstoppable 6th mass extinction: a call for urgent international action. J Popul Sustain 1(2):67–81. https://doi.org/10.3197/jps.2017.1.2.67
Newman J (2012) Control of algae with barley straw. https://nora.nerc.ac.uk/id/eprint/19957/1/BarleyStrawtocontrolalgae.pdf. Accessed 18 Mar 2023
Newman JR, Barrett PRF (1993) Control of Microcystis aeruginosa by decomposing barley straw. J Aquat Plant Manage 31:203–203
Ó hUallacháin D, Fenton O (2010) Barley (Hordeum vulgare)-induced growth inhibition of algae: a review. J Appl Phycol 22:651–658. https://doi.org/10.1007/s10811-009-9492-z
Oduro C, Hatcher PE, Newman J (2005) Controlling Azolla filiculoides using Artemisia dracunculus and A. vulgaris leaf extracts. In: International Symosium on Intractable Weeds and Plant Invaders, Ponta Degada, Azores, Portugal, Programme Abstracts, p.34
Pandey DK (1994) Inhibition of salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hysterophorus L.). I. Effect of leaf residue and allelochemicals. J Chem Ecol 20:3111–3122. https://doi.org/10.1007/BF02033714
Pęczuła W, Suchora M (2014) The influence of barley straw extract addition on the growth of duckweed (Lemna valdiviana Phil.) Under laboratory conditions. Knowl Manage Aquat Ecosyst (415):01. https://doi.org/10.1051/kmae/2014025
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52(3):273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Computer programme. https://www.R-project.org/. Accessed 4 Apr 2023
Rajabi H, Filizadeh Y, Soltani M, Fotokian MH (2010) The use of barley straw for controlling of cyanobacteria under field application. J Fish Aquat Sci 5(5):394–401. https://doi.org/10.3923/jfas.2010.394.401
Roberts J, Florentine S (2022) A global review of the invasive aquatic weed Cabomba caroliniana [A. Gray](Carolina fanwort): current and future management challenges, and research gaps. Weed Res 62(1):75–84. https://doi.org/10.1111/wre.12518
Schrader KK (2005) Evaluation of several commercial algicides for control of odor-producing cyanobacteria. J Aquat Plant Manage 43:100–102
Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8(1):14435. https://doi.org/10.1038/ncomms14435
Simberloff D (2021) Maintenance management and eradication of established aquatic invaders. Hydrobiologia 848:2399–2420. https://doi.org/10.1007/s10750-020-04352-5
Tasker SJ, Foggo A, Bilton DT (2022) Quantifying the ecological impacts of alien aquatic macrophytes: a global meta-analysis of effects on fish, macroinvertebrate and macrophyte assemblages. Freshw Biol 67(11):1847–1860. https://doi.org/10.1111/fwb.13985
Xiao X, Huang H, Ge Z, Rounge TB, Shi J, Xu X et al (2014) A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti‐cyanobacterial activities. Environ Microbiol 16(5):1238–1251. https://doi.org/10.1111/1462-2920.12226
Zehnsdorf A, Hussner A, Eismann F, Rönicke H, Melzer A (2015) Management options of invasive Elodea nuttallii and Elodea canadensis. Limnologica 51:110–117. https://doi.org/10.1016/j.limno.2014.12.010
Zhu X, Dao G, Tao Y, Zhan X, Hu H (2021) A review on control of harmful algal blooms by plant-derived allelochemicals. J Hazard Mater 401:123403. https://doi.org/10.1016/j.jhazmat.2020.123403
Acknowledgements
The following study was fully funded by the ID-UB “Excellence Initiative - Research University”. Therefore, the authors would like to express their deep gratitude for the shown support, which allowed this research to be conducted. We would also like to sincerely thank Adam Mięsikowski, whose involvement in plant measurement was invaluable and reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Financial or non‑financial interests
None.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Online Resource 1
Results of the post-hoc test for morphological data obtained at the end of the experiment. (CSV 8.49 KB)
Online Resource 2
Spreadsheet containing water physicochemical data collected over the duration of the experiment for Cabomba caroliniana. (CSV 9.54 KB)
Online Resource 3
Spreadsheet containing water physicochemical data collected over the duration of the experiment for Elodea nuttallii. (CSV 5.56 KB)
Online Resource 4
Morphological data of Cabomba caroliniana and Elodea nuttallii collected before the start of the experiment. (CSV 8.91 KB)
Online Resource 5
Morphological data of Cabomba caroliniana and Elodea nuttallii collected at the end of the experiment. (CSV 10.7 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Draga, M., Gąbka, M. The beneficial effect of barley straw extract addition on the growth of two aquatic invasive alien species (Elodea nuttallii and Cabomba caroliniana) under laboratory conditions. Biologia 79, 11–21 (2024). https://doi.org/10.1007/s11756-023-01550-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01550-z