Abstract
Amorpha fruticosa L. (Fabaceae) shows two reproductive modes, generative via seeds and vegetative via root-shoots. We studied the reproductive ability on a mixed sample collected from 6 localities in the city of Bratislava: compound fruiting per bush (12.55 ± 8.55), fruiting per bush (70.33 ± 48.04) and fruits per bush (16127.33 ± 24212.25). No significant difference was found between germination values from unpeeled fruits (achenes) and peeled fruits (seeds). Both seeds and fruits germinated very well both immediately after harvest and after storage under different temperature conditions. Peeled achenes germinated earlier. We stored seeds and fruits under different conditions, and found that the highest seed germination values were achieved by seeds stored in the dark at 22°C (60.00%) and by non-stored seeds (57.50%). The longest seedlings grew from seeds (8.48 mm) and from fruits (9.08 mm) stored at 22°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the currently accepted taxonomic concept, the genus Amorpha L. (Fabaceae) includes 16 species native to North America occurring in various habitats, especially along rivers, savannas and prairies (Wilbur 1975; Straub et al. 2009; Straub and Doyle 2014). Some Amorpha taxa have a relatively limited distribution and are rare or endemic, and in contrast, some species occur in large sympatric geographic areas (cf. Straub and Doyle 2014).
From a taxonomy point of view, the genus is regarded as notoriously difficult. It is interesting that because of the very complicated taxonomy, problems with distinguishing of taxa and enormous morphological and ecological variation (e.g., Rydberg 1919; Palmer 1931; Wilbur 1975; Isely 1998), a comprehensive phylogenetic study focusing on Amorpha was not provided until Straub and Doyle (2014). Based on molecular markers (plastid and nuclear data), the authors described a complicated picture of evolution and suggested phylogenetic hypotheses justifying the range of variability in the genus Amorpha.
In the genus Amorpha, only one taxon, A. fruticosa L., has become naturalised worldwide and invaded the territories of Africa, South America, Asia and Europe (Straub et al. 2009; POWO 2023). From the point of view of taxonomy, A. fruticosa is one of the most complex taxa of the genus. In both native and adventive ranges, it is known as a taxon with enormous morphological and environmental plasticity. This also reflects the fact that the species name has from 16 to at least 39 synonyms depending on the taxonomic treatment (Palmer 1931; Wilbur 1975; Straub and Doyle 2014; POWO 2023). Wilbur (1975) pointed out the taxonomic complexity of A. fruticosa and considered this species to be a complex of taxa with problematic delimitation and interrelationships. Similar conclusions were reached by Straub and Doyle (2014), who, based on a study of a North American population, revealed that A. fruticosa shares the same or similar plastid haplotypes of different sympatric Amorpha species due to processes such as hybridisation and introgression. This gene flow combined with phenotypic plasticity may be one of the reasons for its great morphological diversity and ability to invade different types of habitats. Nevertheless, as these authors stated, the issue requires further study. Similarly, the taxonomy of European Amorpha populations has not been resolved, and currently, all populations are assigned to the species A. fruticosa (Straub and Doyle 2014; POWO 2023).
Amorpha fruticosa is a deciduous shrub from the family Fabaceae, usually with purple inflorescence, and with flowers created only by single (wrapped around) petal (banner petal). The fruit is a pod with 1–2 well-germinating seeds. It was introduced to Slovakia in 1850 (Medvecká et al. 2012), it has been planted in parks since the second half of the 19th century (Chrtková 1988). Until now, its occurrence is known from the warmest parts of Slovakia: Borská nížina lowland, Podunajská nížina lowland and Východoslovenská nížina lowland (Mereďa et al. 2021). In Slovakia, A. fruticosa is registered by the “Invasive Organisms Act” as an alien invasive plant with European significance (Government Regulation No. 449/2019 Coll. n.d.). It has high reproductive capacity, creates monocultures in habitats, thereby displacing the original flora, changing successional patterns and reducing biodiversity (Mereďa et al. 2021, Iamonico 2022). The species can be observed when driving on the motorway or around main roads in the warm parts of Slovakia, as well as in the warmest areas of Europe. Because it clearly tolerates soil salinity and summer droughts, it is an almost unbeatable species for the middle dividing strip of motorways (Bulíř 1988). The focus of this work was to draw attention to this shrub, which is registered as invasive in almost all European countries (Seebens et al. 2017; Roy et al. 2020; EPPO 2021), but it is not considered a dangerous invasive species of Europe (European Commission 2019, 2022). A. fruticosa is covered by Slovak legislation; it is registered as a non-native invasive species of Slovak importance (Government Regulation No. 449/2019 Coll. n.d.). Land owners are obliged to dispose of this species on their land and cultivating/spreading the species is a criminal offense (Act No. 150/2019 n.d.). Fortunately, A. fruticosa is not very widespread in the natural communities of Slovakia, so there is a high probability for its successful elimination from the open landscape.
In order to implement management measures, it is necessary to know the reproductive cycle of the species and the amount of offspring (seeds) that the plant is able to produce annually. With seeds, it is essential to know their germination immediately after ripening, or the ability to germinate after some time of storage. For this reason, we focused on the number of flowers, fruits and seeds produced from one bush. Furthermore, germination at the time of maturity and germination of seeds stored in different conditions. This study should contribute to the knowledge of the reproductive abilities of A. fruticosa and serve to support preventive environmental measures. The objectives of the study are 1.) to determine the reproductive capacity and generative potential of A. fruticosa and 2.) to contribute to the knowledge of seed germination from fruits stored under different conditions.
Materials and methods
Material collection
The study was conducted in the Bratislava city region (Slovak Republic) in 2022. The city lies on the border of the Podunajská nížina Lowland and the Malé Karpaty Mts (Mazúr and Lukniš 1986). Its geological bases are fluvial sediments, granites and granodiorites in the territory of the Malé Karpaty Mts (Polák et al. 2011; Maglay et al. 2018). Fluvisols predominate, and with the transition to the Malé Karpaty Mts, they are replaced by Cambisols (Šály and Šurina 2002). The territory belongs to a warm climate region (Lapin et al. 2002) with an annual rainfall of 500–700 mm (Faško and Šťastný 2002). Potential vegetation in the area is associated with willow-poplar floodplain woods (Salicion albae (Oberd. 1953) Th. Müller et Görs1958) and elm floodplain woods (Ulmenion Onberd. 1953), while Carpathian oak-hornbeam woods (Carici pilosae-Carpinenion betuli J. et M. Michalko ined.) is common on the slopes of the Malé Karpaty Mts (Michalko and Berta 1985).
A. fruticosa is a deciduous shrub flowering from May to June, with usually purple flowers (and yellow anthers) arranged into spike inflorescences (and infrutescence). Although the flowers in Fabaceae typically have five petals, in Amorpha species, only one petal (banner petal) is typical, and the other four are missing (Wilbur 1975). The populations of A. fruticosa from which we obtained samples correspond to this general characteristic.
For the detection of reproductive capacity and seed germination, fruits from populations growing in six various localities were collected (Table 1). The basic methodological approach was taken from the work of Pérez-Harguindeguy et al. (2013). One shrub was selected in each locality, that was not broken, did not suffer from any disease, showed good fitness and was fertile (mature fructification stage, all fruits brown). Than, in this shrub, three branches were selected from the middle of the plant and measured (base circumference, length), than all compound infrutescences were harvested. Compound infrutescences were immediately analysed for the number of individual infrutescences and the number of fruits. These functional traits were established as follows: 1.) morphological: shrub height, crown projection, number of branches per shrub, branch circumference, branch length, 2.) regenerative: number of compound infrutescences per shrub, infrutescences per shrub, and fruits per shrub.
After the end of the experiment, all plant material (mainly fruits and seeds) was discarded to prevent the unwanted spread of this invasive alien species.
Seed and fruit preparation
Fruits from all six populations were mixed into one complete set. Then, six random fruit sets were selected from the complete set (the weight of one set was ca. 150 g) and stored according to three regimes: non-stored, stored in the dark at 22°C, and stored in the dark at 8°C. The non-stored set was used for germination tests immediately after collection. Both stored sets were tested for germination after 20 days of storage. Fruits from fruit sets were peeled by hand; those sets changed to seed sets. Each fruit contained one seed.
The seeds and fruits stored at 22°C were weighed on a laboratory scale (Sartorius, balance sensitivity 0.001 g).
The seed germination test was conducted according to the design shown in Table 2, where seeds (S) and fruits (F) were stored under different conditions: non-stored (N), in the dark at 22°C (R), and in the dark at 8°C (F).
Water absorbency
Twenty seeds and twenty fruits stored in the dark at 22°C for 20 days were used to determine the water absorbency (WA). Each individual seed/fruit was weighed (w0), soaked in distilled water for 24 hours, and then weighed again (w). All measurements were repeated five times. The following equation for water absorbency was used: WA = (w - w0)/w0.
Seed germination
Fifty seeds/fruits were taken from each of the three fruit sets and three seed sets and were divided into five plastic Petri dishes (90 mm in diameter) containing four layers of filter paper (KA 0/80) and 6 ml of deionised water. All seeds/fruits were cultivated in a cell box (in the dark at 22°C) for 15 days. The five Petri dishes (20 seeds/fruits per dish) represented five replicates. A radicle protrusion of 2 mm was the criterion for seed germination.
The number of germinated seeds and seedling length were measured on the 1st, 2nd, 4th, 6th, 8th, and 10th day after emergence of the first radicle. Seed germination (%) was obtained as the ratio of germinated seeds to the total number of seeds/fruits.
Data analysis
The obtained data were analysed with a statistical package STATISTICA software (StatSoft Inc. 2013). The observed characteristics were presented as mathematical means and standard deviations (SD) calculated from the measured values.
One-way analysis of variance (ANOVA) was used to evaluate the difference among tested sets in relation to the early growth. The dependent variables were seed germination and seedling length; independent categorical variables were groups including combinations of generative organ, storage type (see Table 2) and day of reading (2nd, 4th, 6th day for seeds; 6th, 8th, 10th day for fruits). Data were presented from the Tukey HSD test.
Difference between water absorbencies of seeds and fruits was estimated by t-test. All statistical tests were performed at the significance level of 0.05.
Results
Five basic morphometric parameters and three reproductive parameters are presented in Table 3. During the peeling of the seeds from the fruits, it was found that each fruit contained exactly one seed. For that reason, the number of fruits obtained corresponds to the average number of seeds produced by one shrub. Large variability was found for the number of fruits per shrub. Water absorbency values for seeds and fruits are presented in Table 4. Fruits had bigger water absorbancy (0.014 g) than seeds (0.008 g), but no significant differences were found (P < 0.05).
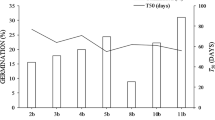
All dehulled seeds began to germinate on the second day after establishment of the experiment. On the sixth day of cultivation, seed germination for individual sets of seeds reached its maximum, and further monitoring is therefore not shown (Fig. 1). The highest seed germination values were achieved by seeds stored in the dark at 22°C (60.00%) and non-stored seeds (57.50%). Seeds stored in the dark at 8°C germinated much less (27.50%).
Seed germination (%) of A. fruticosa after different type of storage (see Table 2)
Sprouts began to appear in all fruits on the sixth day after the establishment of the experiment. On the tenth day of cultivation, seed germination for individual fruit sets reached its maximum, and further monitoring was therefore not necessary (Fig. 1). The highest seed germination values were achieved by fruits stored at 22°C (40.00%), followed by non-stored fruits (32.50%), and the lowest values were achieved by fruits stored at 8°C (22.50%). A complete comparison of the differences between individual treatments (see Table 2) is presented in Table 5. It was found that the compared seed/fruit (respective germination days and storage type) did not differ significantly in germination (Table 5). The dynamics of changes over time for all seed/fruit sets are shown in Fig. 1.
Seedling growths over time and type of storage is shown in Table 6 and Fig. 2. Significant differences between seeds and fruits (respective germination days and storage type) were not observed. In contrast, significant differences were recorded within some different stored fruits (P < 0.05) (Table 6). The longest seedlings grew from seeds (8.48 mm) and from fruits (9.08 mm) stored at 22°C. The lowest seedling length value was found from fruits stored at 8°C (3.43 mm).
Seedling length (mm) of A. fruticosa after different types of storage (see Table 2)
Discussion
A biopotential and bioproductivity study of A. fruticosa (Krpan et al. 2011, 2015) was conducted in Forestry Sunja (FA Sisak, Croatia) to determine the possibility of its use in forestry. The study found that biomass production of A. fruticosa was directly correlated with the number of sprouts per unit area. Additionally, there was a reduction in height gain (compared to the first year), an increase in mean stem diameter from 2 to 3 mm, stem branching, and flowering and fruiting in the second year. So, the older the bush, the more wood it brings, but also more seeds. This finding is expected. Although we measured the size of the branches in our study, we did not work with a sufficient amount of data to determine these dependencies in populations growing in Bratislava. During locality visits, we noticed that some of the shrubs (not young shrubs) did not bloom and therefore did not participate in the generative potential of the population. We have no explanation for this state, but we will address this phenomenon in the future.
In a study of five populations of A. fruticosa from different sites in Bulgaria, Kozuharova et al. (2020) reported fruit/infrutescence and infrutescence/shrub numbers of 152 ± 15 and 436 ± 157, respectively. This corresponds to 66272 fruits/shrub. Our result obtained from Bratislava (central Europe, Slovakia) was an average of 16127 fruits/shrub (with high variability, Table 3), which is significantly less. Shrubs in these climates are also shorter (Krpan et al. 2011). It can be assumed that A. fruticosa is a relatively thermophilic species, and thus, the area of Bulgaria may be more suitable for its vitality and reproductive capacity. In agreement, Krpan et al. (2011) reported numerous stands of A. fruticosa in warm lowland forests (Genisto elatae-Quercetum roboris Ht. 1938) in Croatia (southern Europe).
The fruits of A. fruticosa are achenes that are difficult to peel. In nature, therefore, generative propagation will be realized by fruits rather than seeds. The experiment conducted investigated both germination from seeds and germination from unpeeled fruits (Fig. 1). The values of germination and initial growth from fruits were found to be comparable to those from seeds (Figs. 1, 2).
During our fieldwork, we observed a small population of adult bruchids Acanthoscelides pallidipennis (Motschulsky, 1874) on the A. fruticosa shrubs. This beetle originates from North America and is the main pest of A. fruticosa (Szentesi 1999). The first collection of this species in Europe dates from 1972 (Hungary and Bulgaria) and 1975 (former Yugoslavia) (Borowiec 1980, 1983; Szentesi 1999). It was first found in Slovakia in 1988 (Strejček 1991). Adult bruchids feed on pollen and nectar of flowering plants and do not consume host tissue. They lay their eggs on immature fruits containing only developing seeds (Szentesi et al. 2017). A. pallidipennis in the larval stage destroys the inside of the pod of A. fruticosa by feeding (Szentesi 1999). The highest recorded percentage of seed infestation in Hungary is 61% (Szentesi 1999), while in the United States (California), where A. fruticosa and A. pallidipennis are native, infestation reaches 87% (Rogers and Garrison 1975). Thus, the presence of this beetle can affect annual fruit production of A. fruticosa. A. pallidipennis represents a very important bio-agent for the control of generative reproduction in the invasive species A. fruticosa in Europe. Study of the population dynamics between A. fruticosa and A. pallidipennis should be aim of the future research.
Yücedağ and Gültekin (2011) found that seed germination of A. fruticosa without storage was 64.73%. Our results also show that untreated seeds germinate very well immediately after collection and without pre-treatment (Fig. 1). These results agree with that of an older study (Dirr and Heuser 1987). The fruits began to germinate 3 days later than the seeds. The pericarp delayed the onset of germination but did not limit it (Fig. 1). The water absorbency of the fruits was twice that of the seeds (Table 4).
If the seeds were kept in open jars under room conditions (at approximately 20°C), then increasing the sowing times after collection resulted in a significant decrease in seed germination (Yücedağ and Gültekin 2011). The delayed start of the germination test (storage length, delayed planting) had an effect on seed/fruit germination in our experiments (Fig. 2). This likely means that A. fruticosa seeds are germinating after ripening but gradually lose this ability and become dormant. The presence of dormant seeds in A. fruticosa was confirmed by Cox and Klett (1984), who reported that stratification at 3 to 4°C for 2 and 8 weeks increased the germination rate; 30 min of scarification in sulfuric acid reduced germination by 50%. The application of different pre-treatments would be better to obtain good germination. In our experimental plots, we noted vegetative growth but not growth from seeds. Therefore, it is likely that many of the fruits will become part of the soil seed bank.
The results of our experiments confirmed the spreading potential of A. fruticosa. Pre-requisites for the spread of the species include high production of fruits and seeds and their high germination (Fig. 1). In addition, A. fruticosa is also capable of wide dispersal via vegetative reproduction (Möllerová 2005; Kolyada and Kolyada 2018). Under our local conditions, A. fruticosa spreads mainly in lowland areas of southern Slovakia (Mereďa et al. 2021). Diaspores sources include original plantings near motorways and parks and ornamental plantings, from which diaspores can penetrate into native habitats (Ferus et al. 2020). "The plant is especially easily dispersed within the watersheds of large rivers, where seasonal flooding is regular. Seeds and other propagules are buoyant, and when the water recedes, new plants emerge, forming dense thickets where only a few other species can coexist" (Grabić et al. 2022). The high fruit production of A. fruticosa (Table 3) provides the possibility for active dispersal along riverbeds naturally under fluctuations in the surface water level during spring floods (Shevchyk et al. 2021).
The invasive spread of A. fruticosa is aided by its nitrogen-fixing and allelopathic abilities. Soil enrichment with nitrogen may influence the main ecosystem properties and decrease plant diversity (Boscutti et al. 2020; Pellegrini et al. 2021; Vujanović et al. 2022). Numerous studies have confirmed the high allelopathic potential of A. fruticosa (Csiszár 2009; Novak et al. 2018; Krstin et al. 2020). We know little about the possibilities of spreading this invasive species through seeds; moreover, this species can propagate vegetatively. Therefore, attention should be given to generative reproduction and the possibilities and methods of population spread, including seed dormancy, soil seed banks, and allelopathic abilities in the ecosystem.
Conclusion
Amorpha fruticosa L. (Fabaceae) is a non-native species in Central Europe and is listed as invasive in many countries. It is likely that the invasive strategy of this shrub consists in the large number of achenes produced and the good germination of the seeds. We studied reproductive ability on a mixed sample collected from 6 localities in the city of Bratislava. We found that one bush of A. fruticosa is capable of producing more than 16 thousand achenes in one year and that the germination rate can be up to 60%. Seed/fruit storage under different conditions (storage length and storage temperature) had no significant effect on germination and initial growth. Thus, storage did not reduce seed/fruit germination. The difference between seeds and fruits was noted in the onset of germination, with seeds germinating several days earlier than fruits. The fruits germinated later and their sprout lengths were comparable to those from seeds.
References
Act No. 150/2019 (n.d.) Act on the prevention and management of the introduction and spread of invasive non-native species and on amendments to certain acts. https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2019/150/#paragraf-2.odsek-1. Accessed 23 July 2022
Borowiec L (1980) A new species of Acanthoscelides Schilsky from Bulgaria (Coleoptera, Bruchidae). Pol Pismo Entomol 50(2):167–170
Borowiec L (1983) Survey of seed-beetles of Bulgaria (Coleoptera, Bruchidae). Pol Pismo Entomol 53(1):107–127
Boscutti F, Pellegrini E, Casolo V, De Nobili M, Buchceri M, Alberti G (2020) Cascading effects from plant to soil elucidate how the invasive Amorpha fruticosa L. impacts dry grasslands. J Veg Sci 31:667–677. https://doi.org/10.1111/jvs.12879
Bulíř P (1988) Vegetační doprovody silnic. Aktuality VŠÚOZ, Průhonice
Chrtková A (1988) Amorpha fruticosa L. In: Bertová L (ed) Flóra Slovenska 4/4. Veda, Bratislava, pp 145–146
Cox RA, Klett JE (1984) Seed germination requirements of native Colorado plants for use in the landscape. Plant Propag 30(2):6–10
Csiszár A (2009) Allelopathic effects of invasive woody plant species in Hungary. Acta Silv Lign Hung 5:9–17
Dirr MA, Heuser CW Jr (1987) The reference manual of woody plant propagation: From seed to tissue culture. Varsity Press, Athens
EPPO – European and Mediterranean Plant Protection Organization (2021) Amorpha fruticosa. EPPO Global Database. https://gd.eppo.int/. Accessed 23 July 2022
European Commission (2019) Commision Implementing Regulation (EU) 2019/1262 of 25 July 2019 amending Implementing Regulation (EU) 2016/1141 to update the list of invasive alien species of Union concern. Official J Eur Union L199:1 (Accessed 23 July 2022)
European Commission (2022) Commision Implementing Regulation (EU) 2022/1203 of 12 July 2022 amending Implementing Regulation (EU) 2016/1141 to update the list of invasive alien species of Union concern. Off J Eur Union L186/10. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R1203. Accessed 25 July 2023
Faško P, Šťastný P (2002) Priemerné ročné úhrny zrážok. Atlas krajiny Slovenskej republiky. Ministerstvo životného prostredia, Slovenská agentúra životného prostredia, Bratislava, Banská Bystrica
Ferus P, Hoťka P, Košútová D, Konôpková J (2020) Invasions of alien woody plant taxa across a cluster of villages neighbouring the Mlyňany Arboretum (SW Slovakia). Folia Oecol 47(2):121–130. https://doi.org/10.2478/foecol-2020-0014
Government Regulation No. 449/2019 Coll (n.d.) Regulation of the Government of the Slovak Republic issuing a list of invasive alien species of concern to the Slovak Republic. https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2019/449/20200101. Accessed 23 July 2022
Grabić J, Ljevanić-Mašić B, Zhan A, Benka P, Heilmeier H (2022) A review on invasive false indigo bush (Amorpha fruticosa L.): Nuisance plant with multiple benefits. Ecol Evol 12:e9290. https://doi.org/10.1002/ece3.9290
Iamonico D (2022) Amorpha fruticosa (false indigo-bush), CABI Compendium. CABI International. https://doi.org/10.1079/cabicompendium.5001. Accessed 25 July 2023
Isely D (1998) Amorpha. Native and Naturalized Leguminosae (Fabaceae) of the United States (Exclusive of Alaska and Hawaii). Brigham Young University, Provo, pp 132–144
Kolyada NA, Kolyada AS (2018) Occurrence of Amorpha fruticosa L. in the South of the Russian Far East. Russ J Biol Invasions 9(1):53–56. https://doi.org/10.1134/S2075111718010113
Kozuharova E, Benbassat N, Ionkova I (2020) The invasive alien species Amorpha fruticosa in Bulgaria and its potential as economically prospective source of valuable essential oil. Pharmacia 67(4):357–362. https://doi.org/10.3897/pharmacia.67.e51334
Krpan APB, Tomašić Ž, Palković PB (2011) Biopotential of indigobush (Amorpha fruticosa L.) – second year of investigation. Å umar list 135(13):103–113
Krpan APB, Tomašić Ž, Zežić Ž, Vuletić D (2015) Bioproductivity of indigobush (Amorpha fruticosa L.) in one-year, two-year and four-year rotation. Å umar list 139(3–4):123–135
Krstin L, Katanić Z, Pfeiffer TŽ, Špoljarić Maronić D, Marinčić D, Martinović A, Čamagajevac IÅ (2020) Phytotoxic effect of invasive species Amorpha fruticosa L. on germination and the early growth of forage and agricultural crop plants. Ecol Res 36:97–106. https://doi.org/10.1111/1440-1703.12184
Lapin M, Faško P, Melo M, Šťastný P, Tomlain J (2002) Klimatické oblasti. Atlas krajiny Slovenskej republiky. Ministerstvo životného prostredia, Slovenská agentúra životného prostredia, Bratislava, Banská Bystrica
Maglay J, Fordinál K, Nagy A, Vlačiky M, Šefčík P, Fričovská J, Moravcová M, Kováčik M, Baráth I, Zlocha M (2018) Geologická mapa Podunajskej nížiny – Podunajskej roviny. Ministerstvo životného prostredia, Štátny geologický ústav Dionýza Štúra, Bratislava
Mazúr E, Lukniš M (1986) Geomorfologické členenie SSR a ČSSR. Časť Slovensko; Slovenská kartografia, Slovenská socialistická republika, Bratislava
Medvecká J, Kliment J, Májeková J, Halada Ľ, Zaliberová M, Gojdičová E, Feráková V, Jarolímek I (2012) Inventory of the alien flora of Slovakia. Preslia 84:257–309
Mereďa P jun, Čejka T, Čiampor F, Hrivnák R, Kalivodová M, Kanka R, Májeková M, Pekárik L, Skokanová K, Šingliarová B, Šibík J, Vlachovičová M (2021) Identifikácia a podrobná analýza prienikových ciest introdukcie a neúmyselného šírenia inváznych nepôvodných druhov na územie Slovenskej republiky a na územie EÚ cez územie Slovenskej republiky (Záverečná správa pre dielo 1). Centrum biológie rastlín a biodiverzity Slovenská akadémia vied, Bratislava
Michalko J, Berta J (1985) Geobotanická mapa ČSSR mierky 1: 200 000. 2. Slovenská socialistická republika. Dunajská Streda. Slovenská akadémia vied,Veda, Bratislava
Möllerová J (2005) Notes on invasive and expansive trees and shrubs. J For Sci 51:19–23
Novak N, Novak M, Barič K, Šćepanović M, Ivić D (2018) Allelopathic potential of segetal and ruderal invasive alien plants. J Cent Eur Agric 19(2):408–422. https://doi.org/10.5513/JCEA01/19.2.2116
Palmer EJ (1931) Conspectus of the genus Amorpha. J Arnold Arbor 12(3):157–197. https://doi.org/10.5962/p.185230
Pellegrini E, Boscutti F, Alberti G, Casolo V, Contin M, De Nobili M (2021) Stand age, degree of encroachment and soil characteristics modulate changes of C and N cycles in dry grassland soils invaded by the N2-fixing shrub Amorpha fruticosa. Sci Total Environ 792:148295. https://doi.org/10.1016/j.scitotenv.2021.148295
Polák M, Plašienka D, Kohút M, Putiš M, Bezák V, Filo I, Olšavský M, Havrila M, Buček S, Maglay J, Elečko M, Fordinál K, Nagy A, Hraško Ľ, Németh Z, Broska I (2011) Geologická mapa Malých Karpát. Ministerstvo životného prostredia, Štátny geologický ústav Dionýza Štúra, Bratislava
Pérez-Harguindeguy N, Díaz S, Gamier E et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. https://doi.org/10.1071/BT12225_CO
POWO (2023) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://www.plantsoftheworldonline.org/ Accessed 21 July 2023
Rogers CE, Garrison JC (1975) Seed destruction in indigobush Amorpha by a seed beetle. J Range Manag 28(3):241–242. https://doi.org/10.2307/3897538
Roy D, Alderman D, Anastasiu P et al (2020) DAISIE - Inventory of alien invasive species in Europe. Version 1.7. Research Institute for Nature and Forest (INBO). Checklist dataset https://doi.org/10.15468/ybwd3x. Accessed 25 Jan 2023
Rydberg PA (1919) Amorpha. North Am Flora 24:26–34
Seebens H, Blackburn TM, Dyer EE et al (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8:14435. https://doi.org/10.1038/ncomms14435
Shevchyk TV, Dvirna TS, Shevchyk VL (2021) On the Distribution Pattern of Amorpha fruticosa L. in the Region of the Kanevskaya Hydroelectric Power Plant (Ukraine) in Connection with Hydrochory. Russ J Biol Invasions 12(2):213–218. https://doi.org/10.1134/S2075111721020090
StatSoft Inc (2013) Electronic statistics textbook. Statsoft, Tulsa. http://www.statsoft.com/text-24book/stahme.html
Straub SCK, Bogdanowicz SM, Doyle JJ (2009) Characterization of 12 polymorphic microsatellite markers for Georgia false indigo (Amorpha georgiana Wilbur var. georgiana), an endangered species, and their utility in other dwarf Amorpha L. species. Mol Ecol Resour 9(1):225–228. https://doi.org/10.1111/j.1755-0998.2008.02409.x
Straub SCK, Doyle JJ (2014) Molecular Phylogenetics of Amorpha (Fabaceae): An Evaluation of Monophyly, Species Relationships, and Polyploid Origins. Mol Phylogenet Evol 76:49–66. https://doi.org/10.1016/j.ympev.2014.02.025
Šály R, Šurina B (2002) Pôdy. Atlas krajiny Slovenskej republiky. Ministerstvo životného prostredia, Slovenská agentúra životného prostredia, Bratislava, Banská Bystrica
Strejček J (1991) Faunistic records from Czechoslovakia. Acta Entomol Bohemoslov 88(2):157–160
Szentesi Á (1999) Predispersal seed predation of the introduced false indigo, Amorpha fruticosa L. in Hungary. Acta Zool Acad Sci Hung 45(2):125–141
Szentesi Á, György Z, Jermy T, Kiss B (2017) Seasonal changes in bruchid (Coleoptera: Chrysomelidae: Bruchinae) assemblages along managed highway ecotones. Eur J Entomol 114:488–499. https://doi.org/10.14411/eje.2017.062
Vujanović D, Losapio G, Milić S, Milić D (2022) The Impact of multiple species invasion on soil and plant communities increases with invasive species co-occurrence. Front Plant Sci 13:875824. https://doi.org/10.3389/fpls.2022.875824
Wilbur RL (1975) A revision of the North American genus Amorpha (Leguminosae-Psoraleae). Rhodora 77(811):337–409
Yücedağ C, Gültekin HC (2011) The effect of sowing time on germination of twenty two Leguminosae species. Afr J Agric Res 6(16):3809–3816. https://doi.org/10.5897/AJAR11.880
Acknowledgements
The research was supported by the Scientific Grants Agency of the Ministry of Education, science, research and sport of the Slovak Republic and the Slovak Academy of Sciences (grants VEGA No. 1/0255/23 and partially VEGA No. 1/0007/21). We thank the two anonymous reviewers for their helpful comments and suggestions.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Šerá, B., Žarnovičan, H., Hodálová, I. et al. Reproductive capacity and seed germination after various storage of the invasive alien plant Amorpha fruticosa L. - a case study from Bratislava. Biologia 79, 1–9 (2024). https://doi.org/10.1007/s11756-023-01549-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01549-6