Abstract
Larval diet is one of the key factors in establishing a mass-rearing/production system for Wolbachia-based approaches that promotes high-quality and high-performance adult mosquitoes at a low/reasonable cost. To identify a suitable larval diet for Aedes aegypti infected with the Wolbachia line (wMID) and wild-type lab-established line (MID), four diets with different protein sources (ranging from 42 to 75%) were compared: fish food (TIL), bovine liver powder (COW), porcine powder meal (PIG), and a mix standard laboratory diet (MFOOD). The COW diet for wMID and MID (without affecting survival to the pupal stage) showed a shorter time to pupation, and the average time was 6 to 7 days, respectively. No significant differences were observed on pupation for wMID and MID, which ranged between 92 and 95% and 96–98%, respectively. A larger pupae size was observed among the TIL, COW, and MFOOD diets for wMID; no differences were recorded for MID. With the COW diet, wMID (7.90 ± 0.06) and MID (7.76 ± 0.10) males had shorter development times from LI to emergence. The stability of the Wolbachia infection was not affected by the diets evaluated. Independently of the Wolbachia infection, all diets are suitable for mass-production and maintenance of Ae. aegypti. Overall, no negative impact was observed on the life history traits evaluated. Considering these results, along with the relative costs of the four diets, PIG and TIL are viable choices given their affordability, accessibility of ingredients in the area. These two low-cost and suitable diets could be used for the Ae. aegypti mass-rearing system in Mexico.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Rear and release” Wolbachia-infected mosquitoes, intended to reduce the transmission of mosquito-borne-diseases, is one of the innovative technologies proposed to control Aedes aegypti (Linnaeus, 1762), the main vector of dengue, chikungunya, and Zika (Ritchie and Staunton 2019; PAHO 2019a). Several countries have recently conducted field trials involving Wolbachia-based strategies (including mass-releases of adult mosquitos) targeted at Aedes populations. Australia (Hoffmann et al. 2014; Schmidt et al. 2017; O’Neill 2018), Fiji, Kiribati, New Caledonia, Brazil, Colombia, Mexico, India, Indonesia, Sri Lanka, Vietnam (Nguyen et al. 2015; O’Neill 2018; Martín-Park et al. 2022), Thailand (Kittayapong et al. 2019), China (Zheng et al. 2019; Zeng et al. 2022), and the United States (Mains et al. 2019) are among them.
The implementation of a Wolbachia-based population suppression control program for Ae. aegypti requires the development of a mass-production system of Wolbachia-infected mosquitoes for long-term male release (PAHO 2019b). Larval diet is a critical component in the process of producing males capable of competing with wild-type adult males in both population suppression and replacement programs (Bellini et al. 2007; Bond et al. 2017; Crawford et al. 2020; Beebe et al. 2021). Additionally, the diet should not influence Wolbachia infection (Yeap et al. 2013), and a financially feasible mosquito mass-rearing process is intrinsically linked to the local availability of diet components at a reasonable cost (Puggioli et al. 2013).
The International Atomic Energy Agency (IAEA) and Guangzhou Wolbaki Biotech Co. Ltd. (Wolbaki), which are leading rear and release initiatives for population suppression through the sterile insect technique using radiation (SIT) and the incompatible insect technique using Wolbachia-induced incompatibility (IIT), recommend tuna meal, bovine liver powder, and brewer’s yeast as Ae. aegypti rearing diets. Such diets, which are high in protein, vitamins, and fatty acids, range in price from $18 to $33.00 USD per kilogram (Puggioli et al. 2013; Zhang et al. 2015). Protein concentrates of animal origin are a key component of larval diets, but they are also expensive in some countries and might be difficult to obtain due to import restrictions.
In Mexico, the Ministry of Health collaborated on both replacement and suppression strategies as part of an initial phase of implementation and evaluation for Ae. aegypti biocontrol, with wMel being tested in Baja California (WMP 2021) and wAlbB in Yucatán (Che-Mendoza et al. 2021; Martín-Park et al. 2022). Furthermore, in 2016, the Yucatan state government signed an international collaboration agreement with Universidad Autonoma de Yucatán (UADY) and Michigan State University (MSU) for the development, application, and evaluation of Wolbachia-based strategies to suppress Ae. aegypti urban populations.
The purpose of this study is to compare the effects of different larval diets used to rear mosquitos under laboratory conditions on the life history of Wolbachia-infected and non-infected native lines of Ae. aegypti collected in Merida, Yucatan. These findings support the mass-rearing of Wolbachia-infected mosquitoes during releasing campaigns to suppress native populations of Ae. aegypti in arbovirus transmission endemic areas such as dengue, Zika, and chikungunya.
Materials and methods
Mosquito line and colony maintenance
The Ae. aegypti mosquito lines used in this study were obtained as eggs with ovitraps from the Mexican Ministry of Health’s National Network of Surveillance (CENAPRECE 2015) in several neighborhoods of the Mexican city of Merida, Yucatan State. Wild populations of Ae. aegypti are not naturally infected with Wolbachia bacteria (Kittayapong et al. 2000; Ross et al. 2020). Here, field-collected eggs were reared and colonized under laboratory conditions at the Laboratorio para el Control Biológico de Aedes aegypti (LCB)-UADY and later tested for being Wolbachia-free (hereinafter referred to as MID) by using standard end-point PCR (Puerta-Guardo et al. 2020). A proportion of the eggs from this first collection were sent to MSU’s Department of Microbiology and Molecular Genetics, where they were colonized and kept at the insectary under standard conditions, including an 80 ± 5% humidity, 28 ± 1 °C temperature, and a photoperiod of L12:D12. Wild populations of Ae. aegypti from Merida were backcrossed for 10 generations with a Wolbachia-infected (wAlbB) Ae. aegypti line with complete cytoplasmic incompatibility, as originally described by (Xi et al. 2005). A novel line derived from the backcrossed eggs, (hereinafter referred to as Ae. aegypti wMID) was returned to LCB-UADY for colonization and mass production.
At LCB-UADY, mosquito rearing was carried out following standard operating protocols. Eggs were incubated for 48 h for embryo development before being dried and stored at room temperature (26 ± 1 °C). Larvae were fed with a MixFood diet (Wolbaki Biotech Co, Ltd., Guangzhou, China) (originally designed for Aedes albopictus (Skuse, 1894)) until pupation at 28 ± 1 °C, as previously described by Zhang et al. (2015). Pupae were transferred to 250 mL plastic cups containing 100 mL of water and placed into BugDorm-1Insect Rearing Cages until adults emerged. Adults were maintained under controlled laboratory conditions for temperature at 26 ± 1 °C, 80 ± 5% humidity, and L12:D12 photoperiod cycle. Blood-feeding was prepared on an aluminum plate covered with Parafilm and provided to female adults 5–6 days after eclosion (Carvalho et al. 2014; Zhang et al. 2018).
Study design
Four diets (treatments) with varying protein sources were evaluated and compared in a randomized experimental design, with three replicates per treatment, using the MixFood as a reference diet modified by Zhang et al. (2015) based on Damiens et al. (2014) for both Ae. aegypti MID and wMID lines. Wolbaki larval rearing trays (L × W × H = 58 × 38 × 4 cm) were used as experimental units. The methodology used to evaluate the effects of protein sources (treatments) on larval development and adult size in both groups was adapted from Zhang et al. (2017) for mass-production systems, with some modifications at LCB-UADY, as detailed in the following sections. The experimental design is shown in Fig. 1.
Diets and dosages for larvae
Four different diets were developed using commercially available ingredients, all of which followed the “standard composition” (90% protein source and 10% brewer’s yeast by weight) proposed by previous authors (Puggioli et al. 2013; Zhang et al. 2015). Details of the ingredients are given in the Online resource: Table S1. The protein origin and concentration of the diets evaluated varied, but all of them contained locally available brewer’s yeast powder (Pronat Ultra Iztapalapa, México, CDMX) (Online resource: Table S2). Thus, the diets tested were made by mixing one part brewer’s yeast powder with nine parts animal protein (mix ratio 1:9), as well as the following commercial ingredients: (1) Tilapia fish food (Biofinguerlin, Malta Texo de México S.A. de C.V, Tenancingo Tlaxcala, México), hereafter referred to as TIL diet; (2) Bovine liver powder by (MP BIOMEDICALSTM, Yantai Valiant Fine Chemicals Co., Ltd. China), hereafter referred to as COW diet; (3) Porcine powder meal by PCP del Sureste (PCP del Sureste SA de CV, Umán, Yucatán, México) hereafter referred to as PIG diet, and (4) a mix (ratio 5:3:2) of bovine liver powder NOW, shrimp powder, and yeast powder (hereafter referred to as MFOOD diet) was considered as reference diet (Zhang et al. 2015).
Diets were provided as a slurry at a concentration of 6% (w/v) in accordance with the feeding scheme recommended by Zhang et al. (2017): 1.2 mL (0.18 mg/larvae) on day 1; no food supply on day 2; 2.1 mL (0.32 mg/larvae) on day 3; 3.3 mL (0.51 mg/larvae) on day 4; 3.9 mL (0.60 mg/larvae) on day 5; and 2.7 mL (0.41 mg/larvae) on day 6. Following the initial pupation on day 5, the remaining larvae were returned to the same rearing tray and fed the last dosage.
Development time
Larval development time was calculated as first larval instar (LI) to pupae stage in days (time to pupation). After 5–7 days, eggs from the wMID and MID experimental and control groups were collected on filter paper, sieved through a 60-mesh sieve to remove dead mosquitoes and other impurities on waxed paper, weighed (2,000 eggs; approx., 0.02 g), and transferred into 10 mL of hatching solution (0.5 mL of brewer’s yeast solution [0.09%] diluted in 9.5 mL of water) in Falcon type tubes for 30 min (Zheng et al. 2015).
Groups of 500 first-instar larvae were transferred into trays (L × W × H = 58 × 38 × 4 cm; Wolbaki Biotech Co. Ltd) containing 4 L of water (Zhang et al. 2015, 2017) to obtain a density of 0.125 larvae/mL within the equivalent range densities (0.05 larvae/mL up to 3.6 larvae/mL) used in optimized mass-rearing systems for Ae. aegypti mosquitoes (Bond et al. 2017; Yeap et al. 2013; Maïga et al. 2019). Feeding solution for each diet was calculated following the feeding protocol as described above. Trays were checked daily at 08:00 and 16:00 h to add water lost by evaporation and each tray was refilled if necessary. The sex of pupae was confirmed by examination of the terminalia using a stereomicroscope (Vargas 1968).
Pupation and average time as pupae
The time to pupation was calculated as the time elapsed between the first larval instar (LI) and pupal formation. The day of first pupae appeared was recorded, and the total number of pupae was counted daily for each treatment and replicate. The average time as a pupa was determined from the day the first pupae were recorded until adult emergence (including all individuals of the sample), and it was expressed as the mean of days as a pupa ± the standard deviation (mean ± SD). Pupae were collected using a 40-mesh sieve and then sex sorted (male/female) with a sex sorter machine Wolbaki separator based on Focks (1980). Pupae were counted and placed in 250 mL plastic cups containing 100 mL of water. The percentage of pupation (male and female) was calculated as the total number of pupae divided by the number of LI (500).
Size of pupae
Pupa size (mm) was determined as described by Timmermann and Briegel (1998), by placing the sample on a glass slide and measuring the width of cephalothorax in ventral view using a digital Dino-Lite and DinoCapture 2.0 software version 1.5.30.A. The sample selection of 30 male and 30 female pupae was as suggested by Carvalho et al. (2014) as a quality control procedure per diet (treatment), when highest pupation was recorded (at 6th day).
Duration of the immature stage to adult emergence
The average time LI to pupae plus average duration of the pupal stage was calculated for each treatment and replicate. A total of 60 pupae (30 males and 30 females) were randomly selected from each treatment (diet) when highest pupation was recorded. Pupae were transferred to cylindrical containers filled with 60 mL of water and protected with a mesh covering until emergence. Adult emergence was individually recorded every day for three days after pupation. The average time for adult emergence was calculated in days for each replicate between treatments (mean ± SD). The percentage of adult (male and female) emergence was determined as the number of individuals that emerged in relation to the total number of pupae that were placed in the cylindrical containers. The percentage (male: female) was calculated as the total number of male pupae in relation to the total number of female pupae including all individuals of the sample.
Body size (wing length)
A total of 30 male and 30 female adults were collected when the highest number of pupae was recorded from each treatment using three replicate trays (10 individuals per tray) as previously described by Puggioli et al. (2013). Adult mosquitoes were stored for 10 min at -20 °C for immobilization. Size of the adults was estimated by measuring their right wing, from the axillary incision to the end of the radius vein apex excluding fringe scales, which is a reliable method of body size indication as described by Packer and Corbet (1989), with slight modifications. The wing was cut off from each mosquito, mounted on a microscope slide with a coverslip and was measured with a digital microscope Dino-Lite (AnMo Electronics Corporation, New Taipei City, Taiwan) and DinoCapture 2.0 software version 1.5.30.A.
Detection of Wolbachia infection in Aedes aegypti by qPCR
The presence of Wolbachia DNA in Ae. aegypti after being fed with the different diets was determined by real-time PCR assay. Briefly, DNA extracted from local populations of Ae. aegypti of Yucatan (Puerta-Guardo et al. 2020), previously transinfected with the Wolbachia supergroup B strain (Xi et al. 2005), was used to amplify a DNA fragment (~ 600–700 bp) of the Wolbachia surface protein gene (wsp) using Wolbachia B specific primers (Xi et al. 2005; Puerta-Guardo et al. 2020). DNA sequencing analysis of these DNA fragments (Macrogen Inc., USA) allowed us to design a new set of primer oligomers and one probe using the primer design tool (Geneious R6 6.1.8 software). Primers and probes were manufactured by Integrated DNA technologies (IDT, USA) (Online resource: Table S3).
A total of 160 mosquitoes distributed in four pools of adult female (n = 80 specimens) and male (n = 80 specimens) Ae. aegypti were processed for total genomic DNA extraction using a Blood and Tissue DNEasy Kit (Qiagen, Hilden, Germany). Mosquitoes were divided per each diet into two pools of 10 males and two pools of 10 females of the wild group (MID, n = 40, F4) and two pools of 10 males and two pools of 10 females of the Wolbachia-infected group (wMID, n = 40, F14). Then, samples were analyzed for Wolbachia infection by endpoint and real-time PCR. A real-time PCR protocol was performed using a Rotor-Gene Q series equipped with 36-well carousel, and a Taq DNA Polymerase recombinant kit including nuclease-free water (10 µL), PCR buffer (10X, 2 µL), MgCl2 (50 mM, 2 µL), dNTPs mix (10 mM, 0.5 µL), forward and reverse primers (100 µM, 0.1 µL each), Wolbachia B probe (100 µM, 0.1 µL), Taq DNA polymerase (5 U/µL, 0.2 µL) and extracted DNA template (200 ng per reaction) in a final volume of 20 µL per reaction. Amplification parameters were established as follows: initial denaturation at 95 °C for 5 min; 40 cycles of denaturation at 95 °C for 30 s, Tm annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, which includes acquiring the fluorescence signal (Cycling A Green: Source: 470 nm; Detector: 510; Gain: 4); a final extension step was performed at 72 °C for 5 min. Autogain optimization was performed at 60 °C each round to discard the background signal.
The results are expressed as CT values that are inversely proportional to the Wolbachia DNA concentration in each sample (total genomic DNA loaded per sample: 200 ng). For CT calculation, we used the analysis tool of the Rotor-Gene Q series software v 2.3.1 (Brid 49) to establish a manual threshold based on positive and negative controls. Based on a standard curve (100–0.001 ng) built with a plasmid encoding a fragment of the wsp gene of Wolbachia strain B, CT values below 38 cycles were considered positive. The Ae. aegypti MID and wMID lines were used as a Wolbachia-free control and as positive control, respectively. To confirm the results obtained by real time PCR, the PCR products (~ 179 bp) were separated on a 1% agarose gel stained with SYBR Safe DNA Gel Stain (Invitrogen by Thermo Fisher Scientific, USA) and then visualized using in a ChemiDoc XRS + System (BioRad, Hercules, California, USA) and Image Lab Software.
Data processing and statistical analysis
The statistical analyses were performed using package GraphPad Prism v.7 (GraphPad Software, Inc, Version 6.01, La Jolla, CA, USA; https://www.graphpad.com) and normality test (Shapiro-Wilk) was used to verify the Gaussian distribution of the data. One-way ANOVA followed by a Tukey test (p < 0.05) was applied for the comparison of means between the groups (diets) with respect to MFOOD, the analyses were carried out independently for each mosquito line (wMID and MID) to detect significant differences among the parameters evaluated (development time to pupation, percentage of pupation, average time as pupae and pupae size, percentage of adult emergence). For the wing length, one-way ANOVA followed by Student’s t-test comparisons between groups (wMID and MID) and treatments (diets) were performed to test statistical significance between the means.
Results
Development time
The development time from LI to pupa for both MID and wMID lines (Table 1) differed significantly among diets for males (F3, 8 = 12.32; p = 0.0023 for wMID and F3, 8 = 13.56; p = 0.0017 for MID). For MID line, males fed with COW diet showed a significantly shorter development time of 6.08 days in comparison with MFOOD (Tukey p = 0.0102) and TIL (Tukey p = 0.0022) and for wMID males 6.23 days in comparison with MFOOD (reference diet) (Tukey p = 0.0040) and TIL (Tukey p = 0.0030). The development time for MID females was also significantly reduced with the COW diet compared with the TIL diet (F3, 8 = 6.63; p = 0.0146 and Tukey p = 0.0095). No significant differences were observed for female larval development in wMID.
Pupation and average time as pupae
Here, we compared the percentage of pupation of Wolbachia-infected and non-infected Ae. aegypti reared under laboratory conditions using four distinct larval diets. Overall, no significant differences in pupation were observed for wMID (F3, 8 = 0.2061; p = 0.8893) or MID (F3, 8 = 0.3388; p = 0.7981) at the different diets used in this study. Briefly, in the wMID line, pupation was not different regardless of the diet or the sex of the pupae (F3, 8 = 0.5019; p = 0.6914 for males and F3, 8 = 2.427; p = 0.1406 for females). In contrast, in the MID line, particularly males, some significant differences were detected in the pupation between protein sources, the TIL diet, it shows the longest time for pupae emergence (F3, 8 = 5.108; p = 0.0290). For MID females, no significant differences were observed (F3, 8 = 1.848; p = 0.2167). Overall, the wMID treatments mean percentage of pupation (from LI to pupae) ranged from 93% (1.02) in MFOOD to 95% (3.94) in TIL, 95% (3.75) in COW, and 92% (9.52) in PIG diets. The percentage of pupation in the MID line ranged from 98% (4.04) (MFOOD) to 96% (2.40) TIL, 98% (2.89) COW, and 96% (3.66) PIG. The adult emergence was completed around 43 h for the wMID line and 46 h for the MID line.
Size of pupae
The size of male pupae for the wMID line was significantly larger under TIL, COW, and MFOOD (F3, 116 = 5.081; p = 0.0024) compared with the PIG diet (Tukey p = 0.0016). No significant differences were observed in the size of female pupae among the protein sources tested (F3, 116 = 1.262; p = 0.2907) (Table 2). Regarding the MID line, no significant differences in pupal size were found for males (F3, 116 = 1.595; p = 0.1944) and females (F3, 116 = 2.341; p = 0.0769) among protein sources evaluated (Table 2).
Duration of the immature stage until adult emergence
Adult emergence (development time from LI until the emergence of adults) for both wMID and MID is shown in Table 1. When compared to MFOOD (Tukey p = 0.0035), TIL (Tukey p = 0.0007), and PIG (Tukey p = 0.0157) diets, wMID males fed with the COW diet had a significantly shorter time until emergence (F3, 8 = 16.57; p = 0.0007). (Table 1); significant differences were observed for the COW diet in comparison with MFOOD (Tukey p = 0.0063) and PIG (Tukey p = 0.0489) (F 3, 8 = 8.229; p = 0.0079) among females (Table 1).
For MID, males showed significant differences in development time to adult emergence (F3, 8 = 13.56; p = 0. 0017). The longest development time until adult emergence was 8.45 ± 0.07 days with TIL with respect to MFOOD (Tukey p = 0.0256), COW (Tukey p = 0.0016) and PIG (Tukey p = 0.0045) diets (Table 1). No significant differences were observed among diets for females (F3, 8 = 3.519; p = 0.0687) (Table 1).
The percentage of adult emergence for wMID males ranged from 89 to 93% and no significant differences were detected (F3, 8 = 0.3705; p = 0.7766) between diets (Fig. 2a). For wMID females, the percentage of emergence ranged from 82.33 to 96.67% and significant differences were detected between diets for the group fed with the COW diet (Tukey p = 0.0382) which showed the lowest percentage of emergence (Fig. 1a) (F3, 8 = 4.547; p = 0.0385). For MID, no significant differences were found in the percentage of emerged males (F3, 8 = 2.615; p = 0.1231) or females (F3, 8 = 1.8221; p = 0.2213) with the different diets (Fig. 2b). No significant differences were detected in the sex percentages of both lines of wMID (F3, 8 = 0.2694; p = 0.8458) and MID (F3, 8 = 0.3733; p = 0.7747) for all diets. (Fig. 2c-d).
Percentage of adult emergence under different diets. a Wolbachia-infected (wMID) Ae. aegypti (n = 90), b non-infected Wolbachia-free native line (MID) Ae. aegypti (n = 90). An asterisk denotes a statistically significant difference among diets (ANOVA, Tukey p < 0.05). M: Male, F: Female. Each bar represents the mean ± standard deviations. The sex percentage of adult mosquitos emerged using different diets. c Wolbachia-infected (wMID) (n = 1,500 per diet) and d wild type (Wolbachia-free) native line (MID) of Ae. aegypti (n = 1,500 per diet). Male and female mosquitoes are represented in black and gray bars, respectively. Each bar represents the mean ± the standard deviations
Body size (wing length)
In this study, 30 male and 30 female Ae. aegypti mosquitoes from each experimental group, wMID and MID, were measured to determine their body size under different feeding diets. An initial one-way ANOVA analysis showed significant differences in the wing sizes between the two lines of mosquitoes, wMID (One-Way ANOVA; Male and Female p < 0.0001) or MID (One-Way ANOVA; Male: p < 0.0001; Female: p < 0.0086) (Fig. 3). Further analysis using the student’s t distribution revealed that only males in the MID group fed the TIL diet varied significantly in body size from the MFOOD group. (Mann Whitney test, p < 0.0001****), while significant differences were found between all diets in the wMID group, including the TIL (p < 0.0001****), COW (p = 0.002***), and PIG (p < 0.0001****) compared to the MFOOD (Fig. 2a, b). In contrast, only the TIL diet in the MID group revealed significant differences compared to the MFOOD (p < 0.0001****) (Fig. 2b), whereas no differences were found in the wMID group.
Wing length of Ae. aegypti mosquitoes reared with different diets. a Wing length of Wolbachia-infected (wMID) (n = 30) and non-infected Wolbachia-free (n = 30) Ae. aegypti males and b Wolbachia-infected (wMID) (n = 30) and non-infected Wolbachia-free (n = 30) Ae. aegypti females (n = 30). Each group of the scatter plot data shows the mean ± the standard deviations (shown as error bars). An asterisk denotes a statistically significant difference between groups as determined by one-way ANOVA and the Student t test (Mann-Whitney). Significance differences p < 0.0001****; p < 0.001***; p < 0.01**
Protein source and Wolbachia infection of mass reared Aedes aegypti
To see if the different dietary regimes affected Wolbachia levels in Ae. aegypti mosquitoes, the abundance of the Wolbachia gene wsp was calculated (Ct values) in pools of male and female adult mosquitos after 3 days of feeding on the different diets (Fig. 4a). When the different diets were compared, the levels of Wolbachia infection revealed that there was no statistically significant effect of diets on Wolbachia infection. (One-way ANOVA, p = 0.0626). As expected, no Wolbachia infection was detected in wild type Ae. aegypti collected in the field (Yucatan) and used as a negative control (Fig. 4b).
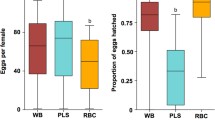
Wolbachia infection in Aedes aegypti reared under different diets. The presence of Wolbachia DNA genome was examined in pools (n = 10 mosquitoes per pool) of Wolbachia-infected (wMID) Ae. aegypti adult mosquitoes using real time PCR. A total of 20 male and 20 female mosquitoes distributed in two pools of ten individuals each were examined per diet (n = 40 mosquitoes per diet). a CT values represent inversely proportional to the Wolbachia DNA concentration in each sample. b Real-time PCR products (~ 179 bp) were separated on a 1% agarose gel stained with SYBR™ Safe DNA Gel Stain. C (+): Ae. aegypti wMID (F9). C (-): Ae. aegypti MID (F4). DNA marker (100 bp). Agarose gel (1%) stained with SYBR safe. M: male; F: female. Statistic differences (p < 0.05) (One-way ANOVA).
Discussion
A series of laboratory experiments were used in this study to evaluate the effects of different protein sources on the artificially maintained life cycle of Ae. aegypti, from larval to adult emergence. We evaluate the parameters in relation to one of the diets, v.gr. MFOOD, which is a recommended diet for Ae. albopictus mass rearing (Zhang et al. 2015); furthermore, when compared to other studies for both Ae. aegypti and Ae. albopictus mass rearing (Puggioli et al. 2013; Carvalho et al. 2014; Bond et al. 2017; Senevirathna et al. 2020). Moreover, the findings might serve as a standard reference for maintaining/rearing Ae. aegypti in laboratory/insectary environments. This information may be obtained through published scientific sources, such as the “Consensus Document on the Biology of the Mosquito Aedes aegypti” (OECD 2018), which serves as a reference.
The optimization of the Aedes mass-production system involves a variety of requirements, including equipment and a balanced diet that promotes high larval survival, fast and homogeneous larval development, pupae and adult homogeneity size, and synchronicity of pupation, all of which result in high-quality (healthy) male adult mosquitoes with high laboratory and field performance in terms of longevity, dispersion (flight ability), mating capacity, fertility, and fecundity (Bellini et al. 2007; Medici et al. 2011; Puggioli et al. 2013; Zhang et al. 2015, 2017; Zheng et al. 2015, 2019; Bond et al. 2017; Kavran et al. 2022).
Larval diet composition should provide a diverse range of nutrients to reduce the risk of nutritional deficiencies, which could have a negative impact on rearing productivity and, ultimately, male mosquito fitness (Timmermann and Briegel 1999). As a result, it must provide high-quality nutrition at a reasonable cost while also ensuring optimum standardized mass-rearing production (Jong et al. 2017). For optimum larval development, insects in general, including mosquitos, require reserves of proteins, amino acids, glycogen, and fatty acids (Clements 1955; Van Handel 1988; Chambers and Klowden 1990; Arrese and Soulages 2010). Overall, the protein sources (particularly PIG, TIL, and MFOOD) had similar effects on development time, pupae number and size, and adult emergence. Only one difference was observed in mosquitoes fed on the PIG diet: they were typically larger in size than those reared with the other diets.
The larval development time for both wMID and MID mosquitoes was 6–7 days (28 ± 1 °C), independent of the protein source utilized for larval feeding. When compared to the other treatments in wMID, only male development time was longer with the TIL diet but similar to the MFOOD diet. This might be attributed to the lower protein concentration (45%) of this animal source, while all evaluated protein sources contain > 50%. Similar studies reported larval development periods of 4–6 days (28 ± 1 °C) (Bond et al. 2017) and/or 5–9 days (27 ± 2 °C) (Gunathilaka et al. 2017), with additional studies reporting similar or longer larval development lengths (Puggioli et al. 2013; Morlan et al. 1963; Chaverri 2001; Cabezas 2005). Aedes aegypti larval development normally lasts 5 to 7 days (OECD 2018), whereas the duration of the Ae. aegypti larval stage in Yucatan has been observed to be an average of 8.1 days under natural conditions (± 2.40 SD) (Manrique-Saide et al. 1998a, b). Our findings revealed that none of the diets tested influenced the development rate of Ae. aegypti larvae.
When compared to a MFOOD diet, COW promoted faster pupation (males) in wMID mosquitoes, whereas PIG and TIL had a similar effect. A similar pattern was observed in Ae. aegypti larvae fed an International Atomic Energy Agency (IAEA) diet containing 25% (wt/wt) bovine liver powder, 50% tuna meal, and 12.5% brewer’s yeast (Damiens et al. 2014). Under standard insectary conditions, the pupal stage lasts between 2.0 and 3.6 days (OECD 2018), whereas the Ae. aegypti pupal stage in Yucatan lasts an average of 3.05 days (± 1.09 SD) (Manrique-Saide et al. 1998a, b).
The size difference between male and female pupae provides the basis for sex separation, especially in the mass production of sterile males for use in SIT, IIT or combined programs. Pupae size is affected by a variety of factors, including larval density, diet, and temperature, among others, and a standardized rearing condition is required to effectively separate males from females, resulting in low female contamination (Papathanos et al. 2009; Yeap et al. 2013; Mikery-Pacheco et al. 2015; Kittayapong et al. 2018; Zacarés et al. 2018; Zheng et al. 2019; Mamai et al. 2020). Also, pupae size has been related to adult wing length in certain studies as the best indicator for adult size in Ae. aegypti (Nasci 1990). This parameter has been considered as one of the quality control processes in mass production systems. The average size of male pupae was comparable to findings from a study of mass-produced genetically modified Ae. aegypti for field release (Carvalho et al. 2014). Significant differences in the sizes of male and female (larger) pupae were detected in this study for both the wMID and MID lines.
In addition, we examined the influence of nutrition on adult mosquito wing size. Previous studies have shown that the size of Ae. aegypti in immature stages is regulated by food availability and population density (Jirakanjanakit et al. 2007; Benedict et al. 2020). Furthermore, proteins play a crucial function as structural components that may regulate mosquito body size (Clements 1992). In this context, mass rearing and release of mosquitoes (reared under high protein composition conditions) enhances the likelihood of successful introductions of Wolbachia-infected Ae. aegypti for mosquito suppression in their natural environments (Yeap et al. 2013). Wing size is one important morphological trait that is genetically correlated to body size (Santos et al. 1992). Among the diets evaluated, nutrition conditions presumably had a substantial influence on wing size. In wMID, mosquitos fed the PIG diet had the largest wing size. As a result of these findings, the PIG diet may show to be a reliable option for providing optimal nutrition throughout larval development and adult mosquito generation in mass rearing systems utilizing readily available components.
The dispersal capacity of Ae. aegypti is also related to adult size (Maciel-de-Freitas et al. 2007). Large adult mosquitos have also been related to short developmental times (Agnew et al. 2002; Gilles et al. 2011; Puggioli et al. 2013), but the contrary has been observed in Ae. albopictus (HC line, triple Wolbachia infection), which does not result in larger adult male mosquitos (Zhang et al. 2015). In this study, developmental time was not related to male and female body size; however, differences in size were influenced by the diet (protein source).
The disparity between adult body size and various larval and pupal measurements (e.g., thoracic length and width, abdominal length and width, and abdominal width) has shown a significant positive correlation with the quantity of food provided during larval development in Ae. aegypti (Gunathilaka et al. 2017). Female body size may influence egg production, mating success, and thus egg production for mass-rearing (Araújo & Gil 2012). Furthermore, reproduction, as well as reduced spermatogenesis and testis size, are affected in small adult males, and females have fewer ovarioles per ovary, resulting in lower fecundity (Medici et al. 2011). All these parameters could be critical to the success of any Wolbachia-based, SIT-based, or combined in vector control programs.
Previous studies have found that rearing Ae. aegypti under Wolbachia infection has no effect on adult wing size (Yeap et al. 2011; Walker et al. 2011; Axford et al. 2016; Ross et al. 2017). In this study, we found the same pattern as in previous studies, where no differences between wMID and MID were observed. These findings show that changes in wing size were caused by nutritional food sources rather than Wolbachia infection. Finally, all the wMID samples tested positive for Wolbachia. As a result, none of the diets provided had any effect on the Wolbachia infection. Wolbachia detection is one of the quality control processes performed inside incompatible insect techniques (IIT) mass-production systems to determine the vertical transmission and stability of Wolbachia (Dobson et al. 2001; Ross et al. 2019).
When compared to natural food sources, commercial animal-based diets have been recommended for producing high-quality adults due to their availability in large quantities and homogeneous composition (nutrients in balance, especially high in protein and essential fatty acids) (Benedict et al. 2009a, b; Damiens et al. 2014; Benedict et al. 2020). The cost of diets developed by the Food and Agriculture Organization in collaboration with the International Atomic Energy Agency (FAO/IAEA) for mosquito mass-rearing is estimated to be US $32 per kilogram for IAEA 1, US $18 for IAEA 2, and US $33 for IAEA 3, all calculated by the Centro Agricultura Ambiente-Italy (CAA) and concluding that IAEA 2 is the preferred choice considering cost-benefits, particularly for adult performance (Puggioli et al. 2013). This diet option might be considered for a mass rearing system if it is accessible and within the overall budget. The estimated cost per kilogram of the diets used in this study was PIG ($2.78), TIL ($3.68), MFOOD (US $67.90), and COW ($116.81). Our findings suggest that the PIG diet has a cost advantage over the other three diets used for Ae. aegypti mass-rearing (depending on area/region and component availability). A PIG-based larval diet requires 16 g for about 6,000 larvae during their developmental cycle. This is the least expensive of the four diets. A TIL diet could potentially be effective in a mass-rearing system for Ae. aegypti.
Overall, the results of this study indicate that independent of Wolbachia infection status, all four diets (same nutritional values) were comparably suitable for rearing and maintaining colonies of Ae. aegypti, with no negative influence on the developmental cycle for both wMID and MID lines. However, based on cost-effectiveness, component availability, and the results provided here, the diets PIG and TIL are the most cost-effective alternatives in the region. These two commercially available low-cost and suitable diets could be used for Ae. aegypti mass-rearing systems (both Wolbachia-infected and non-infected) in Mexico and, potentially, as food for other Aedes species such as Ae. albopictus. It is useful for implementing new technologies such as IIT or a combined IIT-SIT approach.
References
Agnew P, Hide M, Sidobre C, Michalakis Y (2002) A minimalist approach to the effects of density-dependent competition on insect life‐history traits. Ecol Entomol 27:396–402. https://doi.org/10.1046/j.1365-2311.2002.00430.x
Araújo MDS, Gil LHS (2012) Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar J 11:261. https://doi.org/10.1186/1475-2875-11-261
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. https://doi.org/10.1146/annurev-ento-112408-085356
Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA (2016) Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg 94:507–516. https://doi.org/10.4269/ajtmh.15-0608
Beebe NW, Pagendam D, Trewin BJ, Boomer A, Bradford M, Ford A et al (2021) Releasing incompatible males drives strong suppression across populations of wild and Wolbachia carrying Aedes aegypti in Australia. Proc Natl Acad Sci USA 118: e2106828118. https://doi.org/10.1073/pnas.2106828118
Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S (2007) Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial. Area-wide control of insect pests: from research to field implementation. Springer, Netherlands, pp 505–515. https://doi.org/10.1007/978-1-4020-6059-5_47
Benedict MQ, Hood-Nowotny RC, Howell PI, Wilkins EE (2009a) Methylparaben in Anopheles gambiae s.l. sugar meals increase longevity and malaria oocyst abundance but is not a preferred diet. J Insect Physiol 55:197–204. https://doi.org/10.1016/j.jinsphys.2008.11.003
Benedict MQ, Knols BG, Bossin HC et al (2009b) Colonisation and mass rearing: learning from others. Malar J 8:S4. https://doi.org/10.1186/1475-2875-8-S2-S4
Benedict MQ, Hunt CM, Vella MG et al (2020) Pragmatic selection of larval mosquito diets for insectary rearing of Anopheles gambiae and Aedes aegypti. PLoS ONE 15(3). https://doi.org/10.1371/journal.pone.0221838
Bond JG, Ramírez-Osorio A, Marina CF et al (2017) Efficiency of two larval diets for mass-rearing of the mosquito aedes aegypti. PLoS ONE 12(11). https://doi.org/10.1371/journal.pone.0187420
Cabezas C (2005) Dengue en el Perú: aportes para su diagnóstico y control. Rev Peru Med Exp Salud Publica 22:212–228
Carvalho DO, Nimmo D, Naish N et al (2014) Mass production of genetically modified Aedes aegypti for field releases in Brazil. J Vis Exp 83:e3579. https://doi.org/10.3791/3579
Centro Nacional de Programas Preventivos y Control de Enfermedades (CENAPRECE) (2015) Guía metodológica para vigilancia entomológica con ovitrampas. http://www.cenaprece.salud.gob.mx/programas/interior/vectores/descargas/pdf/GuiaMetodologicaVigilanciaEntomologicaOvitrampas.pdf. Accessed 06 July 2022
Chambers GM, Klowden MJ (1990) Correlation of nutritional reserves with a critical weight for pupation in larval Aedes aegypti mosquitoes. J Am Mosq Control Assoc 6:394–399
Chaverri G (2001) Especies de Costa Rica. Aedes aegypti INBIO. Instituto Nacional de Biodiversidad from: http://www.crbio.cr:8080/neoportal-web/species/Aedes%20aegypti. Accessed 12 August 2022
Che-Mendoza A, Martin-Park A, Chávez-Trava JM et al (2021) Abundance and seasonality of Aedes aegypti (Diptera: Culicidae) in two suburban localities of South Mexico, with implications for Wolbachia (Rickettsiales: Rickettsiaceae)-carrying male releases for population suppression. J Med Entomol 58:1817–1825. https://doi.org/10.1093/jme/tjab052
Clements AN (1955) The source of energy for flight in mosquitoes. J Exp Biol 32:547–554. https://doi.org/10.1242/jeb.32.3.547
Clements AN (1992) The biology of mosquitoes. Volume 1. Development, nutrition, and reproduction. Chapman & Hall, London, United Kingdom
Crawford JE, Clarke DW, Criswell V et al (2020) Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol 38:482–492. https://doi.org/10.1038/s41587-020-0471-x
Damiens D, Benedict MQ, Wille M, Gilles JRL (2014) An inexpensive and effective larval diet for Anopheles arabiensis (Diptera: Culicidae): eat like a horse, a bird, or a fish? J Med Entomol 49:1001–1011. https://doi.org/10.1603/ME11289
Dobson SL, Marsland EJ, Rattanadechakul W (2001) Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae). J Med Entomol 38:382–387. https://doi.org/10.1603/0022-2585-38.3.382
Focks DA (1980) An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae). J Med Entomol 17:567–568. https://doi.org/10.1093/jmedent/17.6.567
Gilles JRL, Lees RS, Soliban SM, Benedict MQ (2011) Density-dependent effects in experimental larval populations of Anopheles arabiensis (Diptera: Culicidae) can be negative, neutral, or overcompensatory depending on density and diet levels. J Med Entomol 48:296–304. https://doi.org/10.1603/ME09209
Gunathilaka PA, Uduwawala UM, Udayanga NW, Ranathunge RM, Amarasinghe LD, Abeyewickreme W (2017) Determination of the efficiency of diets for larval development in mass rearing aedes aegypti (Diptera: Culicidae). Bull Entomol Res 108:583–592. https://doi.org/10.1017/S0007485317001092
Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG et al (2014) Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8(9):e3115. https://doi.org/10.1371/journal.pntd.0003115
Jirakanjanakit N, Leemingsawat S, Thongrungkiat S et al (2007) Influence of larval density or food variation on the geometry of the wing of Aedes (Stegomyia) aegypti. Trop Med Int Health 12:1354–1360. https://doi.org/10.1111/j.1365-3156.2007.01919.x
Jong ZW, Kassim NFA, Naziri MA, Webb CE (2017) The effect of inbreeding and larval feeding regime on immature development of Aedes albopictus. J Vector Ecol 42:105–112. https://doi.org/10.1111/jvec.12244
Kavran M, Puggioli A, Šiljegović S, Čanadžić D, Laćarac N, Rakita M et al (2022) Optimization of Aedes albopictus (Diptera: Culicidae) mass rearing through cost-effective larval feeding. Insects 13:504. https://doi.org/10.3390/insects13060504
Kittayapong P, Baisley KJ, Baimai V, O’Neill SL (2000) Distribution and diversity of Wolbachia infections in southeast asian mosquitoes (Diptera: Culicidae). J Med Entomol 37:340–345. https://doi.org/10.1093/jmedent/37.3.340
Kittayapong P, Kaeothaisong NO, Ninphanomchai S, Limohpasmanee W (2018) Combined sterile insect technique and incompatible insect technique: sex separation and quality of sterile Aedes aegypti male mosquitoes released in a pilot population suppression trial in Thailand. Parasit Vectors 11:73–83. https://doi.org/10.1186/s13071-018-3214-9
Kittayapong P, Ninphanomchai S, Limohpasmanee W, Chansang C, Chansang U, Mongkalangoon P (2019) Combined sterile insect technique and incompatible insect technique: the first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl Trop Dis 13(10):e0007771. https://doi.org/10.1371/journal.pntd.0007771
Maciel-de-Freitas R, Codeço CT, Lourenço‐de‐Oliveira R (2007) Body size‐associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Med Vet Entomol 21:284–292. https://doi.org/10.1111/j.1365-2915.2007.00694.x
Maïga H, Mamai W, Bimbilé Somda NS et al (2019) Reducing the cost and assessing the performance of a novel adult mass-rearing cage for the dengue, chikungunya, yellow fever and zika vector, Aedes aegypti (Linnaeus). PLoS Negl Trop Dis 13(9):e0007775. https://doi.org/10.1371/journal.pntd.0007775
Mains JW, Kelly PH, Dobson KL, Petrie WD, Dobson SL (2019) Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes. J Med Entomol 56:1296–1303. https://doi.org/10.1093/jme/tjz051
Mamai W, Maiga H, Somda NSB et al (2020) Aedes aegypti larval development and pupal production in the FAO/IAEA mass-rearing rack and factors influencing sex sorting efficiency. Parasite 27:43. https://doi.org/10.1051/parasite/2020041
Manrique-Saide P, Delfín H, Parra-Tavla V, Ibáñez-Bernal S (1998a) Desarrollo, mortalidad y sobrevivencia de las etapas inmaduras de Aedes aegypti (Diptera: Culicidae) en neumáticos. Rev Biomed 9:84–91
Manrique-Saide P, Ibáñez-Bernal S, Delfín-González H, Parra-Tabla V (1998b) Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tires. Med Vet Entomol 12:386–390
Martín-Park A, Che-Mendoza A, Contreras-Perera Y et al (2022) Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLOS Negl Trop Dis 16(4):e0010324. https://doi.org/10.1371/journal.pntd.0010324
Medici A, Carrieri M, Scholte EJ, Maccagnani B, Luisa Dindo M, Bellini R (2011) Studies on Aedes albopictus larval mass-rearing optimization. J Econ Entomol 104:266–273. https://doi.org/10.1603/EC10108
Mikery-Pacheco O, Serrano-Domínguez K, Marcelín-Chong P, Sánchez-Guillén D (2015) Efficiency of the separation of Aedes (Stegomyia) albopictus (Diptera: Culicidae) male and female pupae using a sieving device. Acta Zool Mex 31:113–115
Morlan HB, Hayes RO, Schoof HF (1963) Methods for mass rearing of Aedes aegypti (L). Public Health Rep 78:711–719. https://doi.org/10.2307/4591909
Nasci RS (1990) Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae). J Med Entomol 27:716–719. https://doi.org/10.1093/jmedent/27.4.716
Nguyen TH, Nguyen HL, Nguyen TY et al (2015) Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 8:1–14. https://doi.org/10.1186/s13071-015-1174-x
O’Neill SL (2018) The use of Wolbachia by the World Mosquito Program to interrupt transmission of Aedes aegypti tTransmitted viruses. In: Hilgenfeld R, Vasudevan S (eds) Dengue and Zika: Control and Antiviral Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1062. Springer, Singapore, pp 355–360. https://doi.org/10.1007/978-981-10-8727-1_24
OECD (2018) Safety assessment of transgenic oOrganisms in the environment. Organization for Economic Co-operation and Development Consensus Document of the Biology of Mosquito Aedes aegypti, Harmonisation of Regulatory Oversight in Biotechnology 8. https://doi.org/10.1787/9789264302235-en
Packer MJ, Corbet PS (1989) Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae). Ecol Entomol 14:297–309. https://doi.org/10.1111/j.1365-2311.1989.tb00960.x
PAHO (2019a) Handbook for Integrated Vector Management in the Americas. Pan American Health Organization, Washington DC. https://iris.paho.org/handle/10665.2/51759. Accessed 10 December 2022
PAHO (2019b) Evaluation of Innovative Strategies for Aedes aegypti Control: Challenges for their Introduction and Impact Assessment. Pan American Health Organization, Washington DC. https://iris.paho.org/handle/10665.2/51375. Accessed 15 December 2022
Papathanos PA, Bossin HC, Benedict MQ, Catteruccia F, Malcolm CA, Alphey L, Crisanti A (2009) Sex separation strategies: past experience and new approaches. Malar J 8:1–8. https://doi.org/10.1186/1475-2875-8-S2-S5
Puerta-Guardo H, Contreras-Perera Y, Pérez-Carrillo S et al (2020) Wolbachia in native populations of Aedes albopictus (Diptera: Culicidae) from Yucatan Peninsula, Mexico. J Insect Sci 20:1–7. https://doi.org/10.1093/jisesa/ieaa096
Puggioli A, Balestrino F, Damiens D et al (2013) Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae). J Med Entomol 50:819–825. https://doi.org/10.1603/ME13011
Ritchie SA, Staunton KM (2019) Reflections from an old Queenslander: can rear and release strategies be the next great era of vector control? Proc R Soc B 286:20190973. https://doi.org/10.1098/rspb.2019.0973
Ross PA, Axford JK, Richardson KM, Endersby-Harshman NM, Hoffmann AA (2017) Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J Vis Exp 126:e56124. https://doi.org/10.3791/56124
Ross PA, Ritchie SA, Axford JK, Hoffmann AA (2019) Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13(4):e0007357. https://doi.org/10.1371/journal.pntd.0007357
Ross PA, Callahan AG, Yang Q et al (2020) An elusive endosymbiont: does Wolbachia occur naturally in Aedes aegypti? Ecol Evol 10:1581–1591. https://doi.org/10.1002/ece3.6012
Santos M, Ruiz A, Quezada-Diaz JE, Barbadilla A, Fontdevila A (1992) The evolutionary history of Drosophila buzzatii. Positive phenotypic covariance between adult fitness components and body size. J Evol Biol 5:403–422. https://doi.org/10.1046/j.1420-9101.1992.5030403.x
Schmidt TL, Barton NH, Rašić G et al (2017) Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol 15(5):e2001894. https://doi.org/10.1371/journal.pbio.2001894
Senevirathna U, Udayanga L, Ganehiarachchi M, Hapugoda M, Ranathunge (2020) Development of an alternative low-cost larval diet for mass rearing of Aedes aegypti mosquitoes. Biomed Res Int 2020:1053818. https://doi.org/10.1155/2020/1053818
Timmermann SE, Briegel H (1998) Molting and metamorphosis in mosquito larvae: a morphometric analysis. Mitt Schweiz Entomol Ges 71:373–387
Timmermann SE, Briegel H (1999) Larval growth and biosynthesis of reserves in mosquitoes. J Insect Physiol 45:461–470. https://doi.org/10.1016/S0022-1910(98)00147-4
Van Handel E (1988) Nutrient accumulation in three mosquitoes during larval development and its effect on young adults. J Am Mosq Control Assoc 4:374–376. https://doi.org/10.5169/seals-402722
Vargas VM (1968) Sexual dimorphism of larvae and pupae of Aedes aegypti (Linn). Mosq News 28:374–379
Walker T, Johnson PH, Moreira LA et al (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. https://doi.org/10.1038/nature10355
WMP (2021) World Mosquito Program. Worldmosquitoprogram.org Mexico. https://www.worldmosquitoprogram.org/es/avances-nivel-mundial/mexico/la-paz. Accessed 18 June 2021
Xi Z, Khoo CC, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326–328. https://doi.org/10.1126/science.1117607
Yeap HL, Mee P, Walker T et al (2011) Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187:583–595. https://doi.org/10.1534/genetics.110.122390
Yeap HL, Endersby NM, Johnson PH, Ritchie SA, Hoffmann AA (2013) Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am J Trop Med Hyg 89:78–92. https://doi.org/10.4269/ajtmh.12-0719
Zacarés M, Salvador-Herranz G, Almenar D et al (2018) Exploring the potential of computer vision analysis of pupae size dimorphism for adaptive sex sorting systems of various vector mosquito species. Parasit Vectors 11:656. https://doi.org/10.1186/s13071-018-3221-x
Zeng Q, She L, Yuan H, Luo Y, Wang R, Mao W et al (2022) A standalone incompatible insect technique enables mosquito suppression in the urban subtropics. Commun Biol 5:1419. https://doi.org/10.1038/s42003-022-04332-6
Zhang D, Zheng X, Xi Z, Bourtzis K, Gilles JRL (2015) Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 10(4):e0121126. https://doi.org/10.1371/journal.pone.0121126
Zhang D, Zhang M, Wu Y et al (2017) Establishment of a medium-scale mosquito facility: optimization of the larval mass-rearing unit for Aedes albopictus (Diptera: Culicidae). Parasit Vectors 10:569. https://doi.org/10.1186/s13071-017-2511-z
Zheng ML, Zhang D, Damiens D, Lees RS, Gilles JRL (2015) Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae) - II - egg storage and hatching. Parasit Vectors 8:348. https://doi.org/10.1186/s13071-015-0951-x
Zheng X, Zhang D, Li Y et al (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572:56–61. https://doi.org/10.1038/s41586-019-1407-9
Zhang D, Li Y, Sun Q et al (2018) Establishment of a medium-scale mosquito facility: tests on mass production cages for Aedes albopictus (Diptera: Culicidae). Parasit Vectors 11:189. https://doi.org/10.1186/s13071-018-2750-7
Acknowledgements
This research was funded by grant YUC2017-03-01-556 awarded to Pablo Manrique-Saide and Abdiel Martín-Park by the Fondo Mixto Consejo Nacional de Ciencia y Tecnología (CONACYT) (México)-Gobierno del Estado de Yucatán and grant AID-OAA-F-16-00082 awarded to Zhiyong Xi and Pablo Manrique-Saide by the U.S. Agency for International Development (USAID). Abdiel Martín-Park is supported by the Investigadoras e Investigadores por México-CONACYT program. We thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) Project: “Fortalecimiento de la Capacidad Nacional para la Producción Masiva de Aedes aegypti con Wolbachia para su Acceso e Implementación como parte del Manejo Integrado de Vectores en México”, which awarded a postdoctoral fellowship No. 2321445 to Yamili Contreras-Perera under supervision of Pablo Manrique-Saide. Special thanks to Suzanna Shugert (suzanna.shugert@gmail.com) for grammatical corrections on the manuscript.
Funding
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: YCP, AMP, PMS. Data Curation: YCP, HPG. Formal Analysis: YCP, HPG. Funding Acquisition: PMS. Investigation: YCP, JFP, SPC. Methodology: YCP, JFP, AMP, HPG, EGM, PMS. Project Administration: PMS, AMP. Resources: PMS, GAT. Software: YCP, HPG. Supervision: PMS, AMP, YCP. Visualization: PMS, YCP, AMP. Writing – Original Draft Preparation: YCP, AMP, HPG, SPC, PMS. Writing – Review & Editing: PMS, AMP, GVP, ACM, HH, FCM, GAT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Contreras-Perera, Y., Flores-Pech, J.P., Pérez-Carillo, S. et al. Different larval diets for Aedes aegypti (Diptera: Culicidae) under laboratory conditions: in preparation for a mass-rearing system. Biologia 78, 3387–3399 (2023). https://doi.org/10.1007/s11756-023-01469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01469-5