Abstract
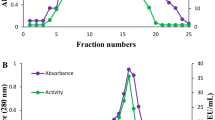
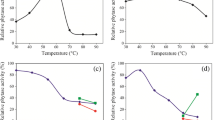
In this study, Lactobacillus brevis isolated from kashar cheese was identified by 16s rRNA method, phytase enzyme from bacteria was partially purified and characterized. Work continued in two directions. The first step was the isolation and identification of the bacteria. In the second step, the phytase enzyme from L. brevis was partially purified and the optimum pH and temperature values of the purified enzyme were determined. The phytase activity of the identified L. brevis was determined as 212.97 U/mL. The molecular mass of the enzyme was determined as 49.9 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method. The Km and Vmax values for sodium phytate were 0.0154 mM and 2.00 µmol/min, respectively. The optimum pH and temperature values of partially purified phytase were determined as pH 3.0, 60 °C, respectively.






Similar content being viewed by others
Availability of data and material
All of the datasets created and/or analyzed during the current study are presented in the article.
Code Availability
Not.
Abbreviations
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- rRNA:
-
Ribosomal RNA
- LAB:
-
Lactic acid bacteria
- PA:
-
Phytic acid
- GRAS:
-
Generally recognized as safe
- DNA:
-
Deoxyribonucleic acid
- PCR:
-
Polymerase chain reaction
- UV:
-
Ultraviolet
- NCBI:
-
National center for biotechnology information
- kDa:
-
Kilodalton
- AC:
-
Accession number
- BLASTn:
-
Basic local alignment search tool
References
Abdolshahi A, Yancheshmeh BS, Arabameri M, Marvdashti LM (2021) Phytase from Bacillus sp. Strain LA12: isolation, purification and characterization. JMBFS 10(4):572–576. https://doi.org/10.15414/jmbfs.2021.10.4.572-576
Ahire JJ, Jakkamsetty C, Kashikar MS, Lakshmi SG, Madempudi RS (2021) In vitro evaluation of probiotic properties of Lactobacillus plantarum UBLP40 isolated from traditional indigenous fermented food. Probiotics Antimicrob Proteins 13(5):1413–1424. https://doi.org/10.1007/s12602-021-09775-7
Ayivi RD, Gyawali R, Krastanov A, Aljaloud SO, Worku M, Tahergorabi R et al (2020) Lactic acid bacteria: food safety and human health applications. Dairy 1(3):202–232. https://doi.org/10.3390/dairy1030015
Barış Ö (2009) Isolation, Characterization and Diagnosis of Effective Bacteria in Dripstone Formation in Caves in Erzurum Province. Doctoral Thesis. Ataturk University
Batista NN, Ramos CL, de Figueiredo Vilela L, Dias DR, Schwan RF (2019) Fermentation of yam (Dioscorea spp. L.) by indigenous phytase-producing lactic acid bacteria strains. Braz J Microbiol 50(2):507–514. https://doi.org/10.1007/s42770-019-00059-5
Bhagat D, Slathia PS, Raina N, Sharma P (2019) Production of phytase from Lactobacillus paracasei strain and its probiotic profile. Indian J Exp Biol 57:839–851
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Chem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Dailin DJ, Hanapi SZ, Elsayed EA, Sukmawati D, Azelee NIW, Eyahmalay J et al (2019) Fungal phytases: biotechnological applications in food & feed industries. In: Yadav A, Singh S, Mishra S, Gupta A (eds) Recent advances in white biotechnology through fungi. Springer. Cham. 65–99. https://doi.org/10.1007/978-3-030-14846-1_2
De Angelis M, Gallo G, Corbo MR, McSweeney PL, Faccia M, Giovine M, Gobbetti M (2003) Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int J Food Microbiol 87(3):259–270. https://doi.org/10.1016/S0168-1605(03)00072-2
Demir Y, Şenol Kotan M, Dikbaş N, Beydemir Ş (2017) Phytase from Weissella halotolerans: purification, partial characterisation and the effect of some metals. Int J Food Prop 20(2):2127–2137. https://doi.org/10.1080/10942912.2017.1368547
Demir Y, Dikbaş N, Beydemir Ş (2018) Purification and biochemical characterization of phytase enzyme from Lactobacillus coryniformis (MH121153). Mol Biotechnol 60(11):783–790. https://doi.org/10.1007/s12033-018-0116-1
Doğan M, Tekiner İH (2020) Extracellular phytase activites of lactic acid bacteria in sourdough mix prepared from traditionally produced boza as starter culture. Food Health 6(2):117–127. https://doi.org/10.3153/FH200013
Duong QH, Clark KD, Lapsley KG, Pegg RB (2017) Determination of myo-inositol phosphates in tree nuts and grain fractions by HPLC–ESI–MS. J Food Compos Anal 59:74–82. https://doi.org/10.1016/j.jfca.2017.02.010
Fukushima A, Uchino G, Akabane T, Aiseki A, Perera I, Hirotsu N (2020) Phytic acid in brown rice can be reduced by increasing soaking temperature. Foods 10(1):23. https://doi.org/10.3390/foods10010023
Georgiev D, Dobrev G, Shilev S (2018) Purification and properties of a phytase from Candida melibiosica 2491.Emir J Food Agric927–934
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Handa V, Sharma D, Kaur A, Arya SK (2020) Biotechnological applications of microbial phytase and phytic acid in food and feed industries. Biocatal Agric Biotechnol 25:101600. https://doi.org/10.1016/j.bcab.2020.101600
Hong SW, Chu IH, Chung KS (2011) Purification and biochemical characterization of thermostable phytase from newly isolated Bacillus subtilis CF92. J Korean Soc Appl Biol Chem 54(1):89–94
Jatuwong K, Suwannarach N, Kumla J, Penkhrue W, Kakumyan P, Lumyong S (2020) Bioprocess for production, characteristics, and biotechnological applications of fungal phytases. Front Microbiol 11:188. https://doi.org/10.3389/fmicb.2020.00188
Karagöz FP, Demir Y, Kotan M, Dursun A, Beydemir Ş, Dikbaş N (2021) Purification of the phytase enzyme from Lactobacillus plantarum: the effect on pansy growth and macro–micro element content. Biotechnol Appl Biochem 68(5):1067–1075. https://doi.org/10.1002/bab.2026
Karaman K, Sagdic O, Durak MZ (2018) Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT 91:557–567. https://doi.org/10.1016/j.lwt.2018.01.055
Kaur P, Vohra A, Satyanarayana T, Singapore (2021) Developments in Fungal Phytase Research: Characteristics and Multifarious Applications. In: Satyanarayana T, Deshmukh SK, Deshpande MV (eds) Progress in Mycology. Springer. Singapore. https://doi.org/10.1007/978-981-16-3307-2_4
Kour D, Kaur T, Yadav N, Rastegari AA, Singh B, Kumar V et al (2020) Phytases from microbes in phosphorus acquisition for plant growth promotion and soil health. In: Rastegari AA, Yadav AN, Yadav N (eds) Trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam, pp 157–176. https://doi.org/10.1016/B978-0-12-820526-6.00011-7
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lee SJ, Jeon HS, Yoo JY, Kim JH (2021) Some important metabolites produced by lactic acid Bacteria originated from Kimchi. Foods 10(9):2148. https://doi.org/10.3390/foods10092148
Luechai S, Dharmsthiti SC (2010) Cultivation of Lactobacillus plantarum KV1 in whey-containing medium for use as starter in vegetable fermentation. Int J Biotechnol Biochem 6:877–888
Mathur H, Beresford TP, Cotter PD (2020) Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 12(6):1679. https://doi.org/10.3390/nu12061679
Mora-Villalobos JA, Montero-Zamora J, Barboza N, Rojas-Garbanzo C, Usaga J et al (2020) Multi-product lactic acid bacteria fermentations: a review. Fermentation 6(1):23. https://doi.org/10.3390/fermentation6010023
Prescott LM, Harley JP (2002) Harley Prescott: Laboratory Exercises in Microbiology. Fifth Edition
Priyodip P, Prakash PY, Balaji S (2017) Phytases of probiotic bacteria: characteristics and beneficial aspects. Indian J Microbiol 57(2):148–154. https://doi.org/10.1007/s12088-017-0647-3
Reddy CS, Achary V, Manna M, Singh J, Kaul T, Reddy MK (2015) Isolation and molecular characterization of thermostable phytase from Bacillus subtilis (BSPhyARRMK33). Appl Biochem Biotechnol 175(6):3058–3067
Sandez Penidez SH, Velasco Manini MA, Gerez CL, Rollán GC (2020) Partial characterization and purification of phytase from Lactobacillus plantarum CRL1964 isolated from pseudocereals. J Basic Microbiol 60:787–798. https://doi.org/10.1002/jobm.202000236
Sardar R, Asad MJ, Ahmad MS, Ahmad T (2022) Optimization of Phytase production by Bacillus sp. (HCYL03) under solid-state fermentation by using Box-Behnken Design. Braz Arch Biol Technol 65. https://doi.org/10.1590/1678-4324-2022210307
Saribuga E, Dikbaş N, Nadaroğlu H, Senol M (2014) Purification, characterization of phytase enzyme from Lactobacillus acidophilus bacteria and determination of it’s some kinetic properties. J Pur Appl Microbiol 8:91–96
Sharma R, Kumar P, Kaushal V, Das R, Navani NK (2018) A novel protein tyrosine phosphatase like phytase from Lactobacillus fermentum NKN51: Cloning, characterization and application in mineral release for food technology applications. Bioresour Technol 249:1000–1008. https://doi.org/10.1016/j.biortech.2017.10.106
Sharma N, Angural S, Rana M, Puri N, Kondepudi KK, Gupta N (2020) Phytase producing lactic acid bacteria: cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci Technol 96:1–12. https://doi.org/10.1016/j.tifs.2019.12.001
Singh N, Kuhar S, Priya K, Jaryal R, Yadav R (2018) Phytase: the feed enzyme, an overview. Advances in Animal Biotechnology and its applications. Springer, Singapore, pp 269–327. https://doi.org/10.1007/978-981-10-4702-2_17
Song HY, El Sheikha AF, Hu DM (2019) The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci Technol 86:553–562. https://doi.org/10.1016/j.tifs.2018.12.001
Sümengen M, Dincer S, Kaya A (2012) Phytase production from Lactobacillus brevis Turk J Biol 36(5):533–541. https://doi.org/10.3906/biy-1111-2
Sumengen M, Dincer S, Kaya A (2013) Production and characterization of phytase from Lactobacillus plantarum. Food Biotechnol 27(2):105–118. https://doi.org/10.1080/08905436.2013.781507
Tarakçıoğlu S (2016) Isolation and Identification of Thermophilic Bacteria from Water Samples Taken from Erzurum Ilıca Thermal Springs and Purification and Characterization of Lipase Enzyme from Bacillus Thermoamylovorans ST-10 Isolate. Master Thesis, Atatürk University
Temiz A (2010) General microbiology application techniques (turkish). Hatiboglu Publishing House
Vasudevan UM, Jaiswal AK, Krishna S, Pandey A (2019) Thermostable phytase in feed and fuel industries. Bioresour Technol 278:400–407. https://doi.org/10.1016/j.biortech.2019.01.065
Zamudio M, Gonzalez A, Medina JA (2001) Lactobacillus plantarum phytase activity is due to non-specific acid phosphatase. Lett Appl Microbiol 32(3):181–184. https://doi.org/10.1046/j.1472-765x.2001.00890.x
Zou LK, Wang HN, Pan X, Tian GB, Xie ZW, Wu Q et al (2008) Expression, purification and characterization of a phyA m-phyCs fusion phytase. J J Zhejiang Univ Sci B 9(7):536–545
Zuo R, Chang J, Yin Q, Chen L, Chen Q, Yang X et al (2010) Phytase gene expression in Lactobacillus and analysis of its biochemical characteristics. Microbiol Res 165(4):329–335. https://doi.org/10.1016/j.micres.2009.06.001
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Neslihan DİKBAŞ, Sevda UÇAR and Şeyma ALIM. The first draft of the manuscript was written by Neslihan DİKBAŞ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not.
Consent to participate
All authors’ consent was obtained.
Consent for publication
All authors’ consent was obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dikbaş, N., Uçar, S. & Alım, Ş. Purification of phytase enzyme from Lactobacillus brevis and biochemical properties. Biologia 78, 2583–2591 (2023). https://doi.org/10.1007/s11756-023-01403-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01403-9