Abstract
Lichens are one of the most responsive components of the ecosystem to reactive forms of nitrogen. In this work, we selected the lichen genera Cladonia and Usnea, composed of terricolous and epiphytic lichens respectively, and described as sensitive to nitrogen, to test the effects of different doses of nitrogen on lichen physiological parameters (photobiont and mycobiont vitality, chitin quantification, nitrogen content and stable isotopes analysis). The main objectives were to check if the activation of protective mechanisms could be stimulated in case of chronic stress (low nitrogen increase for prolonged time), and, if so, if a toxicity threshold could be identified above which these mechanisms fail. The two lichen genera were generally affected by prolonged exposure to increased nitrogen availability. However, Cladonia rangiformis was able to maintain physiological functioning at the lowest nitrogen doses used, whereas thalli of Usnea become overwhelmed. Moreover, the mycobiont appeared to be more sensitive than the photobiont responding to lower nitrogen doses. Although only studies of longer duration and testing more nitrogen doses will be able to determine an accurate toxicity threshold, these results give important clues on the use of lichens as biomonitors for the establishment of environmental policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among ecological indicators, lichens feature extensively. Their morphological characteristics and their complete dependence on the atmosphere to provide their moisture and nutrients make them very sensitive and thus rapid responders to environmental change related to the climate or to atmospheric pollutants (Nimis et al. 2000; Conti and Cecchetti 2001; Jovan 2008).
This feature has been exploited since the XIX century for biomonitoring purposes and today a huge body of literature is available detailing the use of lichens as indicators.
Traditionally, qualitative or quantitative observations of community composition and changes were used to evaluate environmental quality. However, with a diversity of more than 17000 species of lichens existing on Earth (Lumbsch et al. 2011), looking uncritically to species frequency can be easily considered an oversimplification. Over recent decades an approach based on functional traits, both morpho-anatomical attributes like growth form, photobiont type, and reproductive strategy (e.g. Nelson et al. 2015; Benítez et al. 2018; Koch et al. 2019), and physiological attributes like vitality indexes, photosynthetic performance, water use strategy and nutrient management (e.g. Paoli et al. 2011; Gauslaa 2014; Sujetovienė et al. 2019) has provided a more precise methodology to evaluate the effects of environmental factors on lichens.
Following this approach, lichens can be allocated to “functional groups” characterized by a similar response to abiotic factors like pH, solar irradiation, aridity, and eutrophication among others (Nimis and Martellos 2022).
In particular, lichen response to nitrogen has received much attention due to the increasing relevance of reactive forms of nitrogen as major atmospheric pollutants. Indeed, nitrogen is a fundamental requirement for living beings but its excess results toxic for the ecosystem (Steffen et al. 2015). Lichens, considered one of the most responsive components of the ecosystem to reactive forms of nitrogen (Hauck 2010; Jovan et al. 2012), are not only widely used as ecological indicators for nitrogen pollution (Leith et al. 2005), but also form the backbone of the empirical observations used to establish environmental policies like the nitrogen Critical Loads and Critical Levels (Fenn et al. 2008; Cape et al. 2009).
However, not all lichens respond in the same way to nitrogen. Oligotrophic lichen species are highly sensitive and tend to disappear even with slight increases in nitrogen availability in the environment, whereas nitrophytic lichens tolerate high levels of nitrogen (e.g. Munzi et al. 2014; Nimis and Martellos 2022). Various species of Cladonia, for example, considered highly sensitive to nitrogen, have been used in the development of environmental policies aimed at protecting vulnerable environments, such as bogs (APIS, 2019). In this work, we selected the lichen genera Cladonia and Usnea, composed of terricolous and epiphytic lichens respectively to test the effects of different doses of nitrogen on lichen physiological parameters. While the well-known sensitivity of Usnea species makes them reliable indicators of undisturbed environments, recent studies have questioned the suitability of a Cladonia species, namely C. portentosa, as a reference for nitrogen deposition limits in the atmosphere due to its ability to cope with significant nitrogen availability (Munzi et al. 2020). In light of these findings, we conducted an experiment using C. rangiformis, a widespread species in Mediterranean environment, to test its sensitivity to increased levels of nitrogen.
The main objectives of this work were: i) to check if the activation of protective mechanisms could be stimulated in case of short-term treatment with nitrogen concentrations simulating those leading to chronic effects in sensitive species; ii) to identify a toxicity threshold above which these mechanisms fail; and iii) to contribute to the knowledge about species-specific response in the genus Cladonia comparing C. rangiformis with sensitive Usnea species. We expect one of two scenarios: that both species are strictly sensitive, in which case no protective mechanisms will be activated, or that C. rangiformis, like C. portentosa, is less sensitive than believed so far and that its physiological parameters will stay or will return to control values when the protective mechanisms are activated in response to nitrogen stress.
The results can have consequences in the use of the studied lichens in the establishment of environmental policies in the Mediterranean environment.
Material and methods
Lichen material
Thalli of Cladonia rangiformis and Usnea sp. (Fig. 1) were collected in late winter (2016) respectively on soil and on bark of Quercus suber at Companhia das Lezírias (Samora Correia, Portugal), a public limited liability company and Portugal’s largest agricultural and forestry exploitation. The sampling site is a Mediterranean cork-oak forest (519175 E; 4299714 N), in an area where pristine conditions have been maintained for decades thanks to the exclusion of grazing and agricultural activities from the site. Lichen material was transported to the laboratory in paper bags, roughly cleaned from impurity without damaging the thalli, and kept at room temperature following the natural day/night cycle for one day. Nomenclature was based on the online keys published in ITALIC (Nimis and Martellos 2022).
Thalli were then divided into 4 batches of 10 thalli each, and kept at room temperature in the laboratory, exposed to natural light. Each batch was sprayed until completely hydrated every day for 9 weeks, either with water (control) or 50, 150 and 500 μM NH4Cl solutions. Nitrogen doses were chosen to simulate ecologically relevant concentrations following Munzi et al. (2012). At various intervals during the experiment, as indicated in Figs. 2, 3, 4 and 5, part of the material was collected and underwent a set of physiological analyses. Another part was dried at 60 °C and used to measure the percentage of water in lichen material to calculate the dry weight (DW). Material from C. rangiformis was more abundant allowing for an extra set of destructive analyses.
Chlorophyll a fluorescence
Measurements of chlorophyll a fluorescence emission were taken for all lichen samples, 5 replicates per species, as a marker of lichen vitality. In particular, the physiological indicator of photosynthetic efficiency Fv/Fm was considered, representing the potential quantum yield of primary photochemistry (Maxwell and Johnson 2000). Fv/Fm is a widely used parameter measured in photosynthetic organisms and has been successfully applied to lichens (e.g. Munzi et al. 2014; Wu et al. 2017; Wang et al. 2019).
Measurements were taken in hydrated samples dark-adapted for 10 minutes and then illuminated for 1 s with a saturating 3000 µmol m−2 s−1 light pulse, at room temperature, using the Plant Efficiency Analyzer Handy PEA (Hansatech Instruments).
Mycobiont viability
Triphenyltetrazolium chloride (TTC) reduction to triphenylformazan (TPF) was used to assess the mycobiont viability (Bačkor and Fahselt 2005). This quantitative method was developed to evaluate the physiological condition of intact lichens and cultured symbionts since stressors significantly reduce the capability to produce formazan (Bačkor et al. 2003, 2006).
Approximately 15 mg of lichen material was incubated in the dark for 20 h (room temperature) in 2 mL of 5 mM HEPES buffer solution, 0.6% TTC and 0.005% Triton X 100 (one drop). Samples were then removed and rinsed in distilled water until bubbles of Triton X were produced.
Water-insoluble formazan was extracted with 6 mL of ethanol at 65 °C for 45 min. The supernatant ethanol fraction was carefully collected, and the absorbance read at 492 nm. Results were expressed as absorbance units g−1 (DW). Samples were analyzed in triplicate.
Chitin quantification
One of the mechanisms suggested for lichens to neutralize the harmful effects of nitrogen excess is to store the nitrogen in a non-toxic form (Munzi et al. 2017a). Chitin is a major constituent of fungal cell walls and nitrogen makes up around 6.3% of its mass (Dahlman et al. 2003).
Chitin was quantified using a method adapted from Dahlman et al. (2002). Prior to extraction, the samples of 10–25 mg pulverized, freeze-dried lichen (the youngest part of the thalli, up to the first two cm of the ramifications) were suspended in 1 mL 0.2 M NaOH then incubated on a rotary agitator at room temperature for 6 h; after centrifugation at maximum speed for 15 min, another 1 mL 0.2 M NaOH was added to the pellet before incubation overnight at 100 °C to remove amino acids and proteins. After centrifugation (max. Speed, 15 min), the pellet was suspended in 1 mL 6 M HCl and incubated at 100 °C for 5 h to hydrolyze the chitin; after cooling to room temperature and centrifuging (max. Speed, 15 min), the supernatant, containing any glucosamine, was collected in a new Eppendorf vial, then evaporated under a vacuum. Two hundred microliters of water (Milli-Q), 250 μL FMOC-Cl (15 mM in acetone) derivation reagent, and 50 μL borate buffer (1 M, pH 6.3) were added to the extract. After mild agitation and incubation for 10 min, excess FMOC-Cl was removed by two-phase partitioning with 1 mL heptane, repeated twice, and the samples were injected into the HPLC within 10 min. HPLC-UV analysis was performed with a Shimadzu (Japan) LC-6A pump and a Shimadzu (Japan) SPD-6AV UV-Vis detector; data were recorded and analyzed using generic signal recorder software (Azur, Datalys, France); separation was achieved with a Merck LiChroCART 250–4.6 mm Purospher STAR reversed-phase 18e (5 μM) column kept at 25 °C. The mobile phase was 100% methanol (SIGMA) at a flow rate of 1.4 mL min−1, and detection was performed at the wavelength of maximum absorbance (242 nm), determined by spectrophotometric essays on standard solutions or glucosamine (SIGMA). The injection volume was 20 μL. Samples were analyzed in triplicate.
Chitin was quantified through external calibration, using standard solutions of glucosamine (SIGMA) in water, which was derived as the sample (six levels, encompassing sample chromatographic response range, R2 = 0.9935).
Nitrogen content and stable isotopes analysis
Stable isotope ratio analysis was performed at the Centro de Recursos em Isótopos Estáveis - Stable Isotopes and Instrumental Analysis Facility, at the Faculdade de Ciências, Universidade de Lisboa, Portugal. δ13C and δ15N in the samples were determined by continuous flow isotope mass spectrometry (CF-IRMS) (Preston and Owens 1983), on a Sercon Hydra 20–22 (Sercon, UK) stable isotope ratio mass spectrometer, coupled to a EuroEA (EuroVector, Italy) elemental analyser for online sample preparation by Dumas-combustion. Delta Calculation was performed according to δ = [(Rsample – Rstandard)/Rstandard]*1000, where R is the ratio between the heavier isotope and the lighter one. δ15NAir values are referred to air and δ13CVPDB values are referred to PDB (Pee Dee Belemnite). The reference materials used were USGS-25, USGS-35, BCR-657 and IAEA-CH7 (Coleman and Meier-Augenstein 2014); the laboratory standard used was Wheat Flour Standard OAS/Isotope (Elemental Microanalysis, UK). Uncertainty of the isotope ratio analysis, calculated using values from 6 to 9 replicates of laboratory standard interspersed among samples in every batch analysis, was ≤ 0.1‰. The major mass signals of nitrogen and carbon were used to calculate total nitrogen and carbon abundances, using Wheat Flour Standard OAS (Elemental Microanalysis, UK, with 1.47%N, 39.53%C) as elemental composition reference materials. Nitrogen isotopic signature of chemical used for treatments was NH4Cl − 2.9‰ vAir ± 0.04‰ Three replicates per treatment per species were analysed at each collection time.
Results
Nitrogen uptake in lichen thalli of both species was confirmed by the nitrogen concentration measured in lichen material (Table 1). As expected, nitrogen concentrations increased with duration of exposure and the nitrogen dose provided. Interestingly, the final nitrogen concentrations in the two species were similar for the same nitrogen treatments, but the initial value was lower in C. rangiformis, suggesting a slightly higher uptake rate in this species.
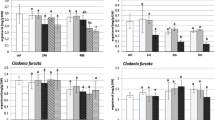
Figure 2 shows the results of chlorophyll a fluorescence measurement for C. rangiformis and Usnea sp. along the 9 weeks of the experiment.
Samples of C. rangiformis showed values similar to the control (> 80% of control) up to day 14 but by the 24th day, the 150 and 500 μM solutions had started affecting lichen health. Dose-related responses were clear after about three weeks: Overall, the lowest dose of treatment (50 μM) tended to stimulate the Fv/Fm parameter by comparison with the control. The 150 μM solution caused a marked decrease in the fluorescence parameter, followed by an increase up to around 80% of the control. The highest dose (500 μM) caused a progressive physiological impairment leading to almost complete inhibition of the photosynthetic system.
In the case of Usnea sp., for all nitrogen doses, an initial slight enhancement was followed by a constant decrease of the Fv/Fm value after day 28. The level of reduction increased with the nitrogen dose/concentration.
Regarding the viability of the mycobiont, as expected, the concentration of TTC remained more or less constant in the control thalli of both species. However, both species displayed an initial increase followed by a decrease in TTC concentration, regardless of the dose of the treatment applied (Fig. 3).
Figure 4 shows how the chitin content varied in treated samples along the treatment period (T0 value is 4.17 mg g−1 for Cladonia and 6.73 mg g−1 for Usnea).
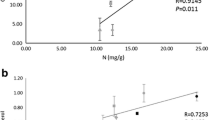
Nitrogen isotopic signature in the samples analyzed tended toward the δ15N of the chemical used for the treatment (−2.9‰) (Fig. 3), with Cladonia changing from −6.34‰ to −3.95‰ and Usnea from −12.8‰ to −4.57‰. Once again, the two species behaved differently. Usnea showed a constant linear relationship along the entire treatment period between its’ nitrogen concentration (showing nitrogen uptake) and the nitrogen isotopic signature (showing nitrogen turnover in the tissue). Conversely, Cladonia went through two phases: the first one, below the threshold value of 1.8% DW, characterized by a rapid nitrogen turnover of nitrogen molecules in the lichen tissue implying an intense metabolic activity, and the second one, above the threshold value of 1.8% DW, where the turnover almost plateaued. Notably, a plateau is reached for the isotopic signature, but not for nitrogen uptake (Table 1; Fig. 5).
Discussion
Atmospheric nutrients, like air-borne nitrogen, are essential for lichens in unpolluted environments (Hauck 2010). An increased nitrogen availability, below the toxicity threshold, can therefore help overcome nutrient limitations, especially for relatively fast-growing lichens, in nutrient-poor sites (Crittenden et al. 1994). The slight, initial increase of Fv/Fm observed in all samples can thus be interpreted as a fertilizing effect, in agreement with previous findings (Carreras et al. 1998; Munzi et al. 2013). However, this beneficial effect was replaced by harmful consequences with the prolonged duration of the experiment and the enhanced amount of nitrogen provided.
Although both considered sensitive, the two species showed some difference in their response to the same amount of nitrogen in their tissue (Table 1), with Usnea sp. being affected by all treatments and C. rangiformis recovering after being treated for some time with the two lowest nitrogen doses. A similar situation was observed in samples of Xanthoria parietina collected from a remote site when compared with samples of the same species collected from a site with high nitrogen availability (Munzi et al. 2013): thalli from the clean site showed impairment while the others were able to recover after exposure to 250 mM (NH4)2SO4. The authors believed the induction of nitrogen tolerance mechanisms during prolonged exposure might explain the difference between the two groups of samples. A recent study on proteomic analysis in Cladonia portentosa reported changes in the protein expression compatible with mechanisms of nitrogen tolerance in samples exposed for more than 10 years to nitrogen manipulation (Munzi et al. 2017b). This suggests that even sensitive species of Cladonia can develop mechanisms to cope with nitrogen stress.
The production of TPF serves as an indicator of metabolic activity and is commonly observed to decline under stress conditions. The observed initial increase in dehydrogenase activity in our samples treated with different concentrations of N may be attributed to the activation of molecular mechanisms, such as chitin production, resulting from increased N availability. This finding is in line with the previously discussed fertilizing effect of nitrogen. However, the subsequent decrease in TPF levels suggests that the toxicity threshold of nitrogen has been exceeded, leading to an adverse impact on the metabolic activity in both species studied.
Chitin production can be seen as a means of neutralizing nitrogen excess. This nitrogen-containing polymer is in fact a main constituent of the fungal cell walls and its production was found to be proportional to the nitrogen available in the environment in lichens and other fungi (Crittenden et al. 1994; Palmqvist et al. 2002). Chitin was found to be associated not only with nitrogen availability, but also with nitrogen tolerance in lichens (Munzi et al. 2017a). Results from Cladonia highlight an increase in chitin content in all treated samples, with similar final content. That suggests that the amount of nitrogen provided already at the lowest dose was enough to saturate the metabolic pathways for chitin production. In Usnea, samples treated with 50 μM increased chitin content reaching a stable value already after two weeks. For 150 and 500 μM the pattern is not so clear, with chitin content lowering or increasing and then lowering. A possible explanation is that with the highest doses of nitrogen, very soon Usnea started suffering a physiological impairment due to nitrogen excess that prevented a clear metabolic response. Therefore, nitrogen storage as chitin seems to be a more effective process in Cladonia than in Usnea.
It has been shown that lichen δ15N varies according to the isotopic signature of the nitrogen source (Munzi et al. 2019). This is confirmed in this study, where the isotopic signature of the chemical used drove the initial lichen isotopic signatures towards less negative values. Possibly, the limited duration of the experiment prevented the nitrogen isotopic signature reaching that of the treatment chemical.
A metabolic shift could be compatible with the activation of protection mechanisms in thalli of Cladonia in response to a prolonged exposure to increased nitrogen availability. Changes in metabolic pathways in response to nitrogen have been described in thalli of C. portentosa, another species considered nitrogen sensitive. In particular, relevant changes in protein expression, affecting transport and regulation of proteins and the energetic metabolism, were observed in the fungal partner after long-term exposure to NH4+ (Munzi et al. 2017b). In our experiments, the shift occurred when the nitrogen concentration in Cladonia tissue reached the value of 1.8% DW. However, more studies are needed before considering it as a general threshold.
The potential ability of lichens of the genus Cladonia to cope with increased nitrogen availability, shown in a recent work by Munzi et al. (2020), through the induction of protection mechanisms would explain the presence of these supposed nitrogen sensitive lichens in polluted areas (Gheza 2015, 2018). On the other hand, the lack of such mechanisms in Usnea confirms and justifies its acknowledged sensitivity to nitrogen.
Conclusions
Physiological tests performed on species of Cladonia and Usnea confirmed that these two lichen genera are generally affected by prolonged exposure to increased nitrogen availability. However, although both included in the functional group of oligotrophic lichens, they responded differently to nitrogen exposure. Our results seem to point at a certain acclimation capacity in Cladonia species when exposed to continuous and gradual increases in nitrogen availability: after an initial impact, changes in metabolism occur that enable Cladonia to maintain physiological functioning at the lowest doses used, whereas thalli of Usnea become overwhelmed. The hypothesis of a higher tolerance in Cladonia than in Usnea is supported by ecological data, namely the presence of Cladonia and the absence of Usnea in relatively polluted areas.
The photobiont’s response seems to start being affected between 150 and 500 μM, while the mycobiont appears to be more sensitive responding between 50 and 150 μM. However, only studies of longer duration and testing more nitrogen doses will be able to determine an accurate toxicity threshold.
References
Air Pollution Information System (APIS) (2019) Critical Loads and Critical Levels - a guide to the data provided in APIS. https://www.apis.ac.uk/critical-loads-and-critical-levelsguide-data-provided-apis. Accessed Jan 2022
Bačkor M, Fahselt D (2005) Tetrazolium reduction as an indicator of environmental stress in lichens and isolated bionts. Environ Exp Bot 53:125–133. https://doi.org/10.1016/j.envexpbot.2004.03.007
Bačkor M, Fahselt D, Davidson RD, Wu CT (2003) Effects of copper on wild and tolerant strains of the lichen photobiont Trebouxia erici (Chlorophyta) and possible tolerance mechanisms. Arch Environ Contam Toxicol 45:159–167. https://doi.org/10.1007/s00244-002-0134-6
Bačkor M, Pawlik-Skowrońska B, Tomko J, Buďová J, Sanità di Toppi L (2006) Response to copper stress in aposymbiotically grown lichen mycobiont Cladonia cristatella: uptake, viability, ergosterol and production of non-protein thiols. Mycol Res 110(8):994–999. https://doi.org/10.1016/j.mycres.2006.05.007
Benítez A, Aragón G, González Y, Prieto M (2018) Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richness and diversity. Ecol Indic 86:18–26. https://doi.org/10.1016/j.ecolind.2017.12.021
Cape JN, van der Eerden LJ, Sheppard LJ, Leith ID, Sutton MA (2009) Evidence for changing the critical level for ammonia. Environ Pollut 157:1033–1037. https://doi.org/10.1016/j.envpol.2008.09.049
Carreras HA, Gudiño GL, Pignata ML (1998) Comparative biomonitoring of atmospheric quality in five zones of Córdoba city (Argentina) employing the transplanted lichen Usnea sp. Environ Pollut 103:317–325. https://doi.org/10.1016/S0269-7491(98)00116-X
Coleman M, Meier-Augenstein W (2014) Ignoring IUPAC guidelines for measurement and reporting of stable isotope abundance values affects us all. Letter to the Editor, Rapid Commun Mass Spectrom 28:1953–1955. https://doi.org/10.1002/rcm.6971
Conti ME, Cecchetti G (2001) Biological monitoring: lichens as bioindicators of air pollution assessment - a review. Environ Pollut 114:471–492. https://doi.org/10.1016/S0269-7491(00)00224-4
Crittenden PD, Kalucka I, Oliver E (1994) Does nitrogen supply limit the growth of lichens. Crypto Bot 4:143–155
Dahlman L, Zetherström M, Sundberg B, Näsholm T, Palmqvist K (2002) Measuring ergosterol and chitin in lichens. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology - culturing, biochemistry, ecophysiology and use in biomonitoring, Springer, pp 348–362. https://doi.org/10.1007/978-3-642-56359-1_21
Dahlman L, Persson J, Näsholm T, Palmqvist K (2003) Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta 217:41–48. https://doi.org/10.1007/s00425-003-0977-8
Fenn ME, Jovan S, Yuan F, Geiser L, Meixner T, Gimeno BS (2008) Empirical and simulated critical loads for nitrogen deposition in California mixed conifer forests. Environ Pollut 155:492–511. https://doi.org/10.1016/j.envpol.2008.03.019
Gauslaa Y (2014) Rain, dew, and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 46:1–16. https://doi.org/10.1017/S0024282913000753
Gheza G (2015) Terricolous lichens of the western Padanian plain: new records of phytogeographical interest. Acta Bot Gall Bot Lett 162(4):339–348. https://doi.org/10.1080/12538078.2015.1108867
Gheza G (2018) Addenda to the lichen flora of the Ticino river valley (western Po plain, Italy). Natural history science. Atti Soc It Sci Nat Museo Civico di Storia Naturale, Milano 5(2):33–40. https://doi.org/10.4081/nhs.2018.381
Hauck M (2010) Ammonium and nitrate tolerance in lichens. Environl Pollut 158:1127–1133. https://doi.org/10.1016/j.envpol.2009.12.036
Jovan S (2008) Lichen bioindication of biodiversity, air quality, and climate: baseline results from monitoring in Washington, Oregon, and California. Gen. Tech. Rep. PNW-GTR-737. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. https://doi.org/10.2737/PNW-GTR-737
Jovan S, Riddell J, Padgett PE, Nash TH III (2012) Eutrophic lichens respond to multiple forms of N: implications for critical levels and critical loads research. Ecol Appl 22(7):1910–1922. https://doi.org/10.1890/03-5368
Koch N, Matos P, Branquinho C, Pinho P, Lucheta F, Ma. de Azevedo Martins S, Ma. Ferrão Vargas V (2019) Selecting lichen functional traits as ecological indicators of the effects of urban environment. Sci Tot Environ 654:705–713. https://doi.org/10.1016/j.scitotenv.2018.11.107
Leith ID, van Dijk N, Pitcairn CER, Wolseley PA, Whitfield CP, Sutton MA (eds) (2005) Biomonitoring methods for assessing the impacts of nitrogen pollution: refinement and testing. Report 386. Joint Nature Conservation Committee, Peterborough, p 290
Lumbsch TH et al (2011) One hundred new species of lichenized fungi: a signature of undiscovered global diversity. Phytotaxa 18:1–127. https://doi.org/10.11646/phytotaxa.18.1.1
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Munzi S, Paoli L, Fiorini E, Loppi S (2012) Physiological response of the epiphytic lichen Evernia prunastri (L.) ach. To ecologically relevant nitrogen concentrations. Environ Pollut 171:25–29. https://doi.org/10.1016/j.envpol.2012.07.001
Munzi S, Branquinho C, Cruz C, Loppi S (2013) Nitrogen tolerance in the lichen Xanthoria parietina: the sensitive side of a resistant species. Funct Plant Biol 40:237–243. https://doi.org/10.1071/FP12127
Munzi S, Cruz C, Branquinho C, Pinho P, Leith ID, Sheppard LJ (2014) Can ammonia tolerance amongst lichen functional groups be explained by physiological responses? Environ Pollut 187:206–209. https://doi.org/10.1016/j.envpol.2014.01.009
Munzi S, Cruz C, Maia R, Máguas C, Perestrello-Ramos MM, Branquinho C (2017a) Intra- and inter-specific variations in chitin in lichens along a N-deposition gradient. Environ Sci Pollut Res 24(36):28065–28071. https://doi.org/10.1007/s11356-017-0378-3
Munzi S, Sheppard LJ, Leith ID, Cruz C, Branquinho C, Bini L, Gagliardi A, Cai G, Parrotta L (2017b) The cost of surviving nitrogen excess: energy and protein demand in the lichen Cladonia portentosa as revealed by proteomic analysis. Planta 245(4):819–833. https://doi.org/10.1007/s00425-017-2647-2
Munzi S, Branquinho C, Cruz C, Máguas C, Leith I, Sheppard L, Sutton M (2019) δ15N in lichens reflects the isotopic signature of ammonia source. Sci Tot Environ 653:698–704. https://doi.org/10.1016/j.scitotenv.2018.11.010
Munzi S, Cruz C, Branquinho C, Cai G, Faleri C, Parrotta L, Bini L, Gagliardi A, Leithg ID, Sheppard LJ (2020) More tolerant than expected: taking into account the ability of Cladonia portentosa to cope with increased nitrogen availability in environmental policy. Ecol Indic 119:106817. https://doi.org/10.1016/j.ecolind.2020.106817
Nelson PR, McCune B, Swanson DK (2015) Lichen traits and species as indicators of vegetation and environment. Bryologist 118(3):252–263. https://doi.org/10.1639/0007-2745-118.3.252
Nimis PL, Martellos S (2022) ITALIC - The Information System on Italian Lichens. Version 7.0. University of Trieste, Dept. of Biology (http://dryades.units.it/italic)
Nimis PL, Scheidegger C, Wolseley PA (eds) (2000) Monitoring with lichens – Monitoring lichens. Nato Science Series, Kluwer Academic Publishers, Dordrecht
Palmqvist K, Dahlman L, Valladares F, Tehler A, Sancho LG, Mattsson JE (2002) CO2 exchange and thallus nitrogen across 75 contrasting lichen associations from different climate zones. Oecologia 133:295–306. https://doi.org/10.1007/s00442-002-1019-0
Paoli L, Pisani T, Guttová A, Sardella G, Loppi S (2011) Physiological and chemical response of lichens transplanted in and around an industrial area of South Italy: relationship with the lichen diversity. Ecotoxicol Environ Saf 74(4):650–657. https://doi.org/10.1016/j.ecoenv.2010.10.011
Preston T, Owens NJP (1983) Interfacing an automatic elemental analyser with an isotope ratio mass spectrometer: the potential for fully automated total nitrogen and nitrogen-15 analysis. Analyst 108:971–977. https://doi.org/10.1039/AN9830800971
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, de Vries W, de Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sörlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:6223. https://doi.org/10.1126/science.1259855
Sujetovienė G, Smilgaitis P, Dagiliūtė R, Žaltauskaitė J (2019) Metal accumulation and physiological response of the lichens transplanted near a landfill in Central Lithuania. Waste Manag 85:60–65. https://doi.org/10.1016/j.wasman.2018.12.017
Wang C-H, Munzi S, Wang M, Jia Y-Z, Tao W (2019) Increasing nitrogen depositions can reduce lichen viability and limit winter food for an endangered Chinese monkey. Basic Appl Ecol 34:55–63. https://doi.org/10.1016/j.baae.2018.10.006
Wu L, Lei Y, Lan S, Hu S (2017) Photosynthetic recovery and acclimation to excess light intensity in the rehydrated lichen soil crusts. PLoS One 12(3):e0172537. https://doi.org/10.1371/journal.pone.0172537
Acknowledgments
The authors are grateful to Companhia das Lezírias (Samora Correia, Portugal) for sampling in its property, Catarina Gouveia for help in the laboratory, and Dr. Lucy J. Sheppard for language revision.
Funding
Open access funding provided by FCT|FCCN (b-on). This research received fundings from the Fundação para a Ciência e Tecnologia (FCT) Investigador grant; the FCT project IF/00964/2013 and the FCT grant UIBD/00329/2020 for the Centre for Ecology, Evolution and Environmental Changes (cE3c).
Author information
Authors and Affiliations
Contributions
SM designed the research. SM, DM and CG collected and analysed the data. SM wrote the article with contributions from DM, CG and CM. CM was responsible for resources and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munzi, S., Graça, C., Martins, D. et al. Differential response of two acidophytic lichens to increased reactive nitrogen availability. Biologia 78, 2049–2057 (2023). https://doi.org/10.1007/s11756-023-01366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01366-x