Abstract
Zinc insufficiency is a nutritional trouble worldwide, especially in developing countries. In the current study, an experiment was conducted to evaluate the effect of supplementation of MS media culture with different concentrations of ZnO nanoparticles (NPs) (0, 10, 20, 40, 80, and 160 ppm) on growth, nutrient uptake, and some physiological parameters of 7-days-old mung bean seedlings. ZnO NPs enhanced the Zn concentration of mung bean from 106.41 in control to more than 4600 µg/g dry weight in 80 and 160 ppm ZnO NPs treated seedlings. Our results showed that ZnO NPs in the concentration range from 10 to 20 ppm had a positive influence on growth parameters and photosynthetic pigments. Higher levels of ZnO NPs negatively affected seedling’s growth by triggering oxidative stress which in turn caused enhancing antioxidative response in seedlings including polyphenol oxidase and peroxidase activity as well as phenolic compounds and anthocyanine contents. Considering the positive effects of ZnO NPs treatment on mungbean seedlings growth, micronutrents, protein and shoot phenolics content, 20 ppm is recommended as the optimal concentration for biofortification. Our findings confirm the capability of ZnO NPs in the remarkable increase of Zn content of mungbean seedlings which can be an efficient way for plant biofortification and dealing with environmental stress.
Similar content being viewed by others
Introduction
It is now apparent that a nutritional deficiency of zinc in humans is widespread, affecting nearly 2 billion individuals worldwide (Prasad 2008). Zinc has a critical role in homeostasis, immune system function, oxidative stress, apoptosis, aging, and significant disorders of great public health interest are associated with zinc deficiency. Reports confirm that dietary supplements of zinc are efficient in the control and treatment of covid 19 (reviewed by Giacalone et al. 2021). Insufficient intake of zinc from food is a major contributor to zinc deficiency in humans which somehow is related to the low Zn content of agricultural soil (Tabrez et al. 2022). Hence there is an urgent need to increase the zinc content and bioavailability in food especially in developing and poor countries (Welch and Graham 2004; Zhao and McGrath 2009).
Crops biofortification offers a sustainable solution to reduce malnutrition and deal with human diseases. Use of nanotechnology to fortify the crops in human societies has received much attention in recent years (El-Ramady et al. 2021; Khan et al. 2021a, b). Crops nano-biofortification could be achieved by seed priming (Rizwan et al. 2019), soil and foliar application (Du et al. 2019; Semida et al. 2021), and cultivation of plants in media rich of candidate nutrient using nanomaterials. In this regard, various aspects should be considered including: (i) finding the appropriate type and shape of nanomaterials, (ii) determination of optimal dose (without negative effects on plant growth and physiology), and (iii) investigation of probable negative or positive effect on the absorption and accumulation of other nutrients.
Mung bean seeds are used up by humans and its hay is used for animal feedstuff that its products can be back to humans through meat, milk, and dairy products. About 6 million-hectare area of the world is under mung bean cultivation (8.5% of the world’s pulse area). Biofortification of crops like mung bean (Vigna radiate (L.) R. Wilczek) which contains carbohydrates, proteins, calcium, β-carotene, iron, and a lot of micronutrients can be a suitable strategy, especially for poor communities to cope with a nutritional deficiency. Haider et al. (2021) and Rani et al. (2022) confirmed the capability of using ZnO NPs for the biofortification of mung beans in the field and hydroponic greenhouse environments. These works have focused on zinc biofortification and failed to address the effect of ZnO NPs treatments on other nutrients uptake. Additionally, more precise studies are required to clarify the effect of ZnO NPs on plant physiology and to find the optimal dose for zinc biofortification.
Mung bean sprouts are now part of the diet of people in many countries as an appetizer and salad. One of the objectives of the current study was to evaluate the capability of the ZnO NPs in mung bean sprout biofortification, performance, and nutrient quality.
Like humans, zinc is crucial for the enzyme functioning in plants and it is very important for plant growth and development. Zn deficiency in plants is one of the major concerns globally. In recent years, the West Asia region has been severely affected by the effects of climate change, such as successive droughts, the expansion of salinized land and dust storms, which have affected agricultural production. Many reports confirm Zn supplementation could alleviate the negative effects of abiotic stresses on plants (Khan et al. 2004; Hussein and Abou-Baker 2018; Thounaojam et al. 2012; Sattar et al. 2021). Micronutrients nanoparticles because of their small size and high surface-to-volume ratio are considered a good candidate for plant uptake and biofortification. The application of appropriate concentrations of zinc oxide nanoparticles (ZnO NPs) with positive effects on plant physiology can improve the growth and protection of different plant species (Mukherjee et al. 2016; Subbaiah et al. 2016; Venkatachalam et al. 2017). A high concentration of ZnO NPs can cause phytotoxicity in plants (Salah et al. 2015; Wang et al. 2016) and produce oxidative stress, which is common in plants in response to environmental stresses. To cope with oxidative stress, plants increase their enzymatic and non-enzymatic anti-oxidants content which helps hinder reactive oxygen species. Some of those non-enzymatic antioxidants are secondary metabolites like phenolic compounds which in turn have nutritional value for humans as antioxidants.
Zinc NPs could be a potential high-performed fertilizer for enhancing plant yield and quality (Du et al. 2019). Accordingly, another aspect of the present study was to investigate the effect of ZnO NPs on the growth and physiology of mung bean seedlings. In the current study, we investigated the effects of different concentrations of ZnO NPs on seedling growth, biochemistry, and physiological response of V. radiate.
Materials and methods
Plant material and culture conditions
Seeds of mung bean (V. radiata) were surface sterilized in 1% (v/v) sodium hypochlorite solution for 15 min, followed by three washes with sterile distilled water. Ten sterilized seeds were placed into Petri dishes containing 10 ml of MS basal medium (Murashige and Skoog 1962), 7% agarose, pH = 5.7 and 0, 10, 20, 40, 80, and 160 ppm of ZnO NPs (10–30 nm, nearly spherical, US-NANO). The stock water suspension of ZnO NPs, before application in media culture, was placed for 30 min in the Elmasonic S80(H) Ultrasonic Cleaner (37 kHz of ultrasonic frequency, of 150 W of effective ultrasonic power) (Elma Schmidbauer GmbH, Singen, Germany) to achieve better dispersion of particles. Petri dishes were placed in a tissue culture room at a temperature of 25 °C, relative humidity of 55%, and a 16-h photoperiod with a light intensity of 2300 lx. Seedlings obtained after 7 days of growth were used for experiments. The biometrical data of 7-days-old seedlings were collected as mean values of the shoot and root fresh and dry weight (mg) and length (cm). For physiological and biochemical assays 7 days old seedlings were frozen in liquid nitrogen and kept at -70 °C and used for the experiments.
Multielement analysis
0.5 g of powdered freeze-dried mung bean shoot samples was placed in polyethylene tube with 8 ml of nitric acid (65%) for 12 h at room temperature. Then 2 ml perchloric acid was added to the samples and kept at 80 °C for 1 h and 150 °C for 3 h. The solution was filtered using Whatman No. 42 filter paper and diluted to 25 mL with ultrapure water and stored in the dark before analysis. ICP-MS (HP-4500, USA) equipped with an Asx-520 auto-sampler was used to define elements concentration.
Pigment contents
The total contents of carotenoids, chlorophylls a and b were measured according to the procedure described by Linchenthaler and Wellburn (1983). Briefly, fresh samples (0.2 g) were homogenized in 80% acetone (Merck, Germany) and the absorbance was recorded spectrophotometrically at 470, 646, and 663 nm. The results were calculated based on the following equations:
Anthocyanin content was extracted using 1% HCl v/v acidified methanol. Fresh samples were homogenized in the extraction solution, centrifuged at 18 000 × g at 4 °C for 15 min, and stored in darkness for 5 h at 5 °C. The amount of anthocyanin was quantified at 550 nm spectrophotometrically (Abdel Latef et al. 2020).
Malondialdehyde and hydrogen peroxide content
Malondialdehyde (MDA) content was determined based on Heath and Packer (1968). Fresh tissues were extracted with 0.1% TCA, and 0.5 ml of the supernatant was mixed with 1 ml of thiobarbituric acid (0.5%) in TCA (20%). After heating the solution at 95 °C for 25 min, the absorbance was read spectrophotometrically at 532 and 600 nm. The amount of MDA was calculated by an extinction coefficient of 155 mM− 1 cm− 1.
For H2O2 quantification, the 0.5 ml of extract (0.1% TCA) was mixed with 0.5 ml potassium phosphate buffer (10 mM, pH 7.0) and 1 ml KI (1 M). The absorbance was measured at 390 nm based on the Velikova et al. (2000) method.
Protein content and antioxidant enzyme activity
0.2 g of fresh samples was ground in 1 M Tris-HCl (pH 6.8) at 4 °C. The supernatant was separated at 12 000 × g centrifugation for 15 min and used for protein and enzyme assays. Protein content was quantified with bovine serum albumin as the standard (Bradford 1976).
Antioxidant enzyme assay
For measurement of peroxidase (POX) activity, 50 µl of the extract was mixed with 0.1 ml benzidine, 0.2 ml H2O2 (3%), and 0.2 M acetate buffer (pH 4.8), and the activity was defined at 530 nm (Abeles and Biles 1991).
Polyphenol oxidase (PPO; E.C. 1.14.18.1) activity was measured based on the method described by Raymond et al. (1993). The reaction mixture contained 0.2 ml of pyrogallol (20 mM), 2.5 ml of 200 mM sodium phosphate buffer (pH 6.8), and 50 µl of enzyme extract. The enzyme activity was recorded at 430 nm.
Total phenolic compounds content
For extraction, 0.4 g of dried powder of samples was placed in 80% (v/v) methanol for 48 h and then was put in an ultrasonic bath for 20 min. After centrifugation (10 min at 11 000 × g), the supernatant was utilized for the detection of total phenolic compounds.
For determination of total phenolic content, 100 µl of the extract was mixed with 500 µl of Folin-Ciocalteu reagent and 400 µl of sodium carbonate. Then, the reaction solution was kept for 30 min at room temperature. The absorbance was read spectrophotometrically at 630 nm (Singleton and Rossi 1965).
Statistical analyses
The experiment was set up in a completely randomized design. For experimental treatment, 10 repetitions were used with 10 seeds each (1 Petri dish). For biometrical analysis at least 20 plants were assayed in each treatment. For physiological and biochemical assays 25 seedlings were pooled and used as one replication of triplicate.
Each data point was an average of three or five replicates. Presented data were analyzed by one way ANOVA (analysis of variance) using SPSS (version 21). The significance of differences was determined according to Duncan’s multiple range tests at the 0.05 level of probability. The graphs were designed with Graphpad Prism version 6 and Microsoft Excel version 2010. Principal component analysis (PCA) and Pearson correlation test were conducted using publicly available Past3.16 software.
Results
Zn content significantly increased with the increase of ZnO NPs concentration in media culture, on average, from 106.41 in control to more than 4 600 µg/g dry weight in 80 and 160 ppm ZnO NPs treated seedlings. No significant difference in Zn content was observed in the samples treated with 10 and those treated with 20 ppm ZnO NPs. Interestingly, with the exception of Zinc, the lowest concentrations of the all assayed elements were observed in 10 ppm ZnO NPs treated samples. On the other hand, the highest levels of calcium, magnesium, phosphorus, manganese, iron, cobalt, copper, and molybdenum were observed in the 40 ppm ZnO NPs treated samples (Table 1). Although none of the evaluated elements showed a positive or negative correlation with zinc, a strong correlation (≥ 90%) between K with Mg and P, Mg with P, Fe and Cu, P with K, Mn with Fe and Co, Fe with P, and Mn and Co were observed (Supplementary Fig. 1).
Seedling growth
ZnO NPs treatment induced changes in the growth biomarkers of V. radiata seedlings (Fig. 1). The use of ZnO NPs in the concentration range from 10 to 20 ppm had a positive influence on growth. The fresh and dry weight of shoot and root increased up to 20 ppm and then decreased at 40, 80, and 160 ppm. Shoot and root length also increased significantly following ZnO NPs treatment up to 20 ppm, while the length of seedlings was negatively affected at higher concentrations. In summary, the highest and lowest rate of seedling biomass including plant height as well as fresh and dry weight was observed in 20 ppm and 160 ZnO NPs treated samples respectively (Supplementary Fig. 2).
Photosynthetic pigments
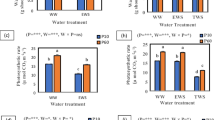
Chl a, Chl b, Chl T and carotenoids content significantly increased (P ≤ 0.05) up to 20 ppm ZnO NPs and then markedly reduced with the increase of ZnO concentrations in media culture (Fig. 2).
The content of photosynthetic pigments: chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in shoots of 7 days old mungbean (V. radiata) seedlings cultured on MS media containing 0, 10, 20, 40, 80 and 160 ppm zinc oxide nanaoparticles. Different letters indicate means that are significantly different at (P ≤ 0.05)
Protein content
The shoot soluble protein content was observed to be the highest at 40 to160 ZnO NPs treated samples (Fig. 3). In the root, total soluble protein content significantly increased up to 40 ppm then significantly decreased under higher concentrations (80 and 160 ppm) of ZnO NPs (P ≤ 0.05).
Lipid peroxidation and H2O2 content
The content of MDA (as a bio-indicator of lipid peroxidation) and H2O2 (as the most stable ROS) in the shoot samples of ZnO treated samples were significantly higher than that of controls (Fig. 4). The highest content of H2O2 and MDA in shoot and root samples treated with ZnO NPs were observed at 160 and 40 ppm respectively. Root H2O2 content in response to ZnO NPs treatment was highly increased as compared to shoot. However, lipid peroxidation in shoot was higher which probably can be justified by higher anti-oxidants in root.
Enzymatic antioxidant assay
ZnO NPs treatment induced the activities of POX and PPO of V. radiate in both shoot and root (Fig. 5). The results showed the activity of POX in the shoot was considerably lower than in root samples. The highest activity levels of POX were observed in the shoot and root samples collected from seedlings grown on 20 ppm and 160 ppm of ZnO NPs, respectively. The highest activity level of PPO activity in both root and shoot was observed at 160 ppm ZnO NPs treated samples.
Non-enzymatic antioxidants
The effect of ZnO nanoparticles on total phenolic compounds and anthocyanin was presented in Fig. 6. The treatment with ZnO NPs increased the total phenolic content of both shoot and root. The highest phenolic compounds induction was observed in shoot and root samples grown on 20 and 160 with the elevation rate of 207% and 160% percent as compared to control. Additionally, the highest level of the shoot and root anthocyanin content was observed at 160 and 80 ppm ZnNPs treated samples respectively (Fig. 6). No significant differences in anthocyanin content were observed in 20 to 160 and 40 to 160 ZnO NPs treated samples of shoot and root respectively.
The principal component analysis (PCA) was performed on Z-scored transformed data of assayed data. PCA showed that 79% variation between samples could be explained by two (PC1 = 57.60 and PC2 = 21.98%) principal components (Fig. 4). The first component (PCA1) separates the 0, 10, and 20 ppm ZnO NPs treated samples from other species primarily based on and shoot zinc, calcium, H2O2, MDA, and chlorophyll content as well as root fresh weight (Fig. 7). The second component (PCA2) separated 40 and 60 ppm ZnO NPs treated samples from other species primarily based on cobalt, potassium, phenolic compounds contents as well as PPO and POX activities. These results confirmed that plant nutritional status is highly affected under ZnO NPs treatment followed by photosynthesis and antioxidative capability.
Discussion
The increased zinc content of shoot in response to ZnO NPs supplementation confirmed that the application of zinc nanoparticles can be an effective way to increase the zinc content of mung bean seedlings. Considering that the recommended daily intake of zinc in the diet is between 8 and 12 mg, with the consumption of 2 g of 7 days old mung bean seedlings, germinated and grown on MS medium containing 80 ppm of zinc oxide nanoparticles, this need can be met. Thus, according to the tremendous effect of ZnO NPs treatment on the zinc content of seedlings (about 45 times more than that of control), this method could lead to an augmentation that could meet the daily needs of adults.
Although the lowest concentration of ZnO NPs used in this study caused a more than 7-fold increase in zinc content of seedlings shoot, however it had a negative effect on the content of other analyzed elements. This finding, along with its positive effect on plant biomass, shows that the positive effect of zinc is related to its own nutritional value, not its effect on the absorption of other elements (at least the elements that have been studied). Because at least a part of the nutrients required for the seedling’s shoot is supplied from the seed storage, it is expected that the content of elements in the shoot samples treated with ZnO NPs should not be less than that of the control samples. Therefore, it seems that the negative effect of zinc (at 10 ppm) on the content of the studied elements is related to its effect on the translocation of elements from seed to shoot not to the absorption of other elements from the medium culture.
Generally, the solubility of Zn in the media culture and soil increases with decrease in pH (Salinitro et al., 2020). It seems that the low pH of MS media culture increased the bioavailability of Zn for plant uptake. In plants, the interaction between Zn and other plant nutrients do exist and both positive and negative interactions are reported (Prasad et al. 2016).
Regarding the positive effect of zinc on plant growth, two points can be mentioned: Firstly, there are some reports that confirm the positive effect of zinc on tryptophan (as a precursor of auxin) and gibberellic acid content in plants which as plant growth regulators can promote plant growth (Mašev and Kutáček 1966; Wang et al. 2021). Secondly, our findings of increased leaves content of Chla, Chlb, and carotenoid (up to 20 ppm) are a good indicator of the positive impact of zinc on plant photosynthesis.
In the current study seedlings’ growth (biomass) showed a strong zinc concentration-dependent trend. The positive impact of low concentration (10 and 20 ppm) and negative impact of high concentration (40 to 160 ppm) of ZnO NP media culture supplementation on seedling growth may be related to its nutritional value on one hand and toxicological effects on the other hand. Leaves photosynthetic pigments as well as root MDA and H2O2 content can be considered as bioindicators for the above-mentioned positive and negative effects.
Zinc is an essential nutrient for plants and it has a role in protein synthesis and activity of several enzymes, as well as membrane integrity, metabolic reactions, water uptake, transport, and gene expressions (Rudani et al. 2018). Hence, an increase in Zn level (at least up to 715% which was observed in 20 ppm ZnO NP supplemented media) was beneficial for the plants, leading to growth improvement. The positive effect of Zn application on plant chlorophyll content and photosynthesis was reported in different plants such as Triticum aestivum L., Brassica juncea (L.) Czern., and Gossypium hirsutum L. (Wu et al. 2015; Khan et al. 2016; Sultana et al. 2016). Similar to our findings the positive impact of low (≥ 20 ppm) concentration of ZnO NPs on plant growth and protein content was reported in Zea mays L. (Sabir et al. 2020), and Lycopersicon esculentum Mill. (Faizan et al. 2018).
The negative effect of a higher concentration of ZnO NPs on plant growth was reported in the other studies (Zhang et al. 2015; Javed et al. 2017; Tymoszuk and Wojnarowicz 2020; Rani et al. 2022). ZnO NP toxicity (at a concentration of 40 to 160 ppm) might be due to the perturbed homeostasis of Zn and its impacts on other elements’ homeostasis (Srivastav et al. 2021). A strong correlation (~ 98%) between shoot Zn and MDA is a signal of lipid peroxidation and probable membrane damage (Supplementary Fig. 1). The finding is an indication of the creation of oxidative stress due to excessive accumulation of zinc as reported in the other plants (Remans et al. 2012; Feigl et al. 2015). Researchers have discussed about the mechanisms of nanoparticle-induced oxidative stress including light activation of electron hole pairs, active electronic configurations and functional groups generation on the surface of nanoparticles, and active redox cycling on the surface of nanoparticle (reviewed by Saliani et al. 2016). There are evidences that confirm the smaller nanoparticles have higher density of surface defects and provide greater free electrons and holes which leads to the generation of ROS (Singh et al. 2021).To eliminate excess ROS, plants activate enzymatic and non-enzymatic antioxidant defense systems. In this regard, the activation of secondary plant metabolism and synthesis of phenolic compounds like anthocyanins is a crucial way to scavenge ROS (Sheteiwy et al. 2016; Mittler 2017; Xu and Rothstein 2018).
In the current study root PPO activity and anthocyanin content showed a significant correlation with internal Zn content (Supplementary Fig. 1). It seems that this strategy could restrain ROS accumulation in the root. A similar response of increased phenolic content to ZnO NP treatment was reported in Brassica nigra (L.) K.Koch, potato, and Coriandrum sativum L. (Marichali et al. 2014; Zafar et al. 2016; Raigond et al. 2017). However, it seems that change in the activity of PPO and POX along with the increased content of phenolic compounds has not been enough to inhibit oxidative damage in the seedling shoot (Wang et al. 2018). This observation is probably due to the fact that the photosynthesis apparatus of plant leaves are among the main site of ROS generation (Khorobrykh et al. 2020).
Comparing the content of assayed minerals in the samples, confirmed that the seedlings grown on the 40 ppm ZnO NPs, accumulated the highest amount of elements (a total of 58175.35 µg/g). Meanwhile, the highest seedling dry-weight biomass was found at 20 ppm ZnO NPs treatment. Considering the content of mineral nutrients in the total biomass produced in 7 day time period, it is determined that the highest mineral level is found in 20 ppm (2118.72) followed by 40 ppm (1602.73), 10 ppm (1324.52), 80 ppm (1308.87), control (1267.81), and 160 ppm (1021.70). These data confirm the advantage of samples grown on 20 ppm ZnO NPs over others.
Conclusion
Taken together our findings confirm the capability of ZnO NP in zinc biofrotification of mung bean seedlings. Despite the significantly increased content of many micro and macronutrients especially under 40 ppm ZnO NPs supplemented medium, its stressful effects were observed as a decrease in growth and photosynthetic pigments and an increase in the content of H2O2 and MDA. Considering the positive effects of ZnO NP on growth, photosynthetic pigments, protein content, activity, and content of enzymatic and non-enzymatic antioxidants in mung bean seedlings, a concentration of 20 ppm is recommended as the optimal concentration.
References
Abdel Latef AAH, Dawood MF, Hassanpour H, Rezayian M, Younes NA (2020) Impact of the static magnetic field on growth, pigments, osmolytes, nitric oxide, hydrogen sulfide, phenylalanine ammonia-lyase activity, antioxidant defense system, and yield in lettuce. Biology 9(7):172. https://doi.org/10.3390/biology9070172
Abeles FB, Biles CL (1991) Characterization of Peroxidases in Lignifying Peach Fruit Endocarp. Plant Physiol 95(1):269–273. https://doi.org/10.1104/pp.95.1.269
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Du W, Yang J, Peng Q, Liang X, Mao H (2019) Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: from toxicity and zinc biofortification. Chemosphere 227:109e116. https://doi.org/10.1016/j.chemosphere.2019.03.168
El-Ramady H, Abdalla N, Elbasiouny H, Elbehiry F, Elsakhawy T, Omara AED et al (2021) Nano-biofortification of different crops to immune against COVID-19: A review. Ecotoxicol Environ Saf 222:112500. https://doi.org/10.1016/j.ecoenv.2021.112500
Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56(2):678–686. https://doi.org/10.1007/s11099-017-0717-0
Feigl G, Lehotai N, Molnár A, Ördög A, Rodríguez-Ruiz M, Palma JM, Kolbert Z (2015) Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann Bot 116(4):613–625. https://doi.org/10.1093/aob/mcu246
Giacalone A, Marin L, Febbi M, Tovani-Palone MR (2021) Current evidence on vitamin C, D, and zinc supplementation for COVID-19 prevention and/or treatment. Electron J Gen Med 18(5):em311. https://doi.org/10.29333/ejgm/11099
Haider MU, Hussain M, Farooq M, Ul-Allah S, Ansari MJ, Alwahibi MS, Farooq S (2021) Zinc biofortification potential of diverse mungbean [V. radiata (L.) Wilczek] genotypes under field conditions. PLoS ONE 16(6):e0253085. https://doi.org/10.1371/journal.pone.0253085
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hussein MM, Abou-Baker NH (2018) The contribution of nano-zinc to alleviate salinity stress on cotton plants. R Soc Open Sci 5(8):171809. https://doi.org/10.1098/rsos.171809
Javed R, Usman M, Yücesan B, Zia M, Gürel E (2017) Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem 110:94–99. https://doi.org/10.1016/j.plaphy.2016.05.032
Khan MK, Pandey A, Hamurcu M, Gezgin S, Athar T, Rajput VD, Gupta OP, Minkina (2021a) Insight into the prospects for nanotechnology in wheat biofortification. Biology 10(11):1123. https://doi.org/10.3390/biology10111123
Khan ST, Malik A, Alwarthan A, Shaik MR (2021b) The enormity of the zinc deficiency problem and available solutions; an overview. Arab J Chem 103668. https://doi.org/10.1016/j.arabjc.2021.103668
Khan R, Gul S, Hamayun M, Shah M, Sayyed A, Ismail H, Gul H (2016) Effect of foliar application of zinc and manganese on growth and some biochemical constituents of Brassica junceae grown under water stress. J Agric Environ Sci 16:984–997. https://doi.org/10.5829/idosi.aejaes.2016.16.5.12923
Khan MU, Qasim M, Jamil M (2004) Effect of Zn on starch content of paddy and zinc content of soil, leaf and root of rice grown in calcareous soils. Int J Agric Biol 6(6):1132–1135
Khorobrykh S, Havurinne V, Mattila H, Tyystjärvi E (2020) Oxygen and ROS in photosynthesis. Plants 9(1):91. https://doi.org/10.3390/plants9010091
Linchenthaler HK, Wellburn AR (1983) Determination of total carotenoides and clorophyll a and b of leaf extracts in diferent solventes. Biochem Soc Transac 11:1591–92. https://doi.org/10.1042/bst0110591
Mašev N, Kutáček M (1966) The effect of zinc on the biosynthesis of tryptophan, andol auxins and gibberellins in barley. Biol Plant 8(2):142–151. https://doi.org/10.1007/BF02930623
Marichali A, Dallali S, Ouerghemmi S, Sebei H, Hosni K (2014) Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind Crops Prod 55:248–257. https://doi.org/10.1016/j.indcrop.2014.02.033
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mukherjee A, Sun Y, Morelius E, Tamez C, Bandyopadhyay S, Niu G, Gardea-Torresdey JL (2016) Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): a life cycle study. Front Plant Sci 6:1242. https://doi.org/10.3389/fpls.2015.01242
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14(5):353–357
Prasad R, Shivay YS, Kumar D (2016) Interactions of zinc with other nutrients in soils and plants-A Review. Ind J Fertilisers 12(5):16–26
Raigond P, Raigond B, Kaundal B, Singh B, Joshi A, Dutt S (2017) Effect of zinc nanoparticles on antioxidative system of potato plants. J Environ Biol 38(3):435–439. https://doi.org/10.22438/jeb
Rani N, Kumari K, Sangwan P, Barala P, Yadav J, Hooda V (2022) Nano-iron and nano-zinc induced growth and metabolic changes in Vigna radiata Sustainability 14(14):8251. https://doi.org/10.3390/su14148251
Raymond J, Rakariyatham N, Azanza JL (1993) Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 34(4):927–931. https://doi.org/10.1016/S0031-9422(00)90689-7
Remans T, Opdenakker K, Guisez Y, Carleer R, Schat H, Vangronsveld J, Cuypers A (2012) Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ Exp Bot 84:61–71. https://doi.org/10.1016/j.envexpbot.2012.05.005
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia ur Rehman M, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277. https://doi.org/10.1016/j.chemosphere.2018.09.120
Rudani L, Vishal P, Kalavati P (2018) The importance of zinc in plant growth-A review. Int Res J Nat Sci 5(2):38–48
Sabir S, Zahoor MA, Waseem M, Siddique MH, Shafique M, Imran M, Muzammil S (2020) Biosynthesis of ZnO nanoparticles using bacillus subtilis: characterization and nutritive significance for promoting plant growth in Zea mays L. Dose-Response 18(3):1559325820958911. https://doi.org/10.1177/1559325820958911
Salah SM, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Jin H (2015) Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep 5(1):1–14. https://doi.org/10.1038/srep14278
Saliani M, Jalal R, Goharshadi EK (2016) Mechanism of oxidative stress involved in the toxicity of ZnO nanoparticles against eukaryotic cells. Nanomed J 3(1):1–14. https://doi.org/10.7508/nmj.2016.01.001
Salinitro M, van der Ent A, Tognacchini A, Tassoni A (2020) Stress responses and nickel and zinc accumulation in different accessions of Stellaria media (L.) Vill. in response to solution pH variation in hydroponic culture. Plant Physiol Biochem 148:133–141
Sattar A, Wang X, Ul-Allah S, Sher A, Ijaz M, Irfan M, Skalicky M (2021) Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress. Saudi J Biol Sci 29(3):1699–1706. https://doi.org/10.1016/j.sjbs.2021.10.061
Semida WM, Abdelkhalik A, Mohamed GF, Abd El-Mageed TA, Abd El-Mageed SA, Rady MM, Ali EF (2021) Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10(2):421. https://doi.org/10.3390/plants10020421
Sheteiwy MS, Fu Y, Hu Q, Nawaz A, Guan Y, Li Z, Hu J (2016) Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ Sci Pollut Res 23(19):19989–20002. https://doi.org/10.1007/s11356-016-7170-7
Singh RD, Kumbhakar P, Pal S, Khannam SK, Kumbhakar P (2021) Investigating morphology-dependent antibacterial property of ZnO nanoparticles and developing an insight into oxidative stress generation and cellular response. Biologia 6(4):1339–1348. https://doi.org/10.1007/s11756-021-00690-4
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer J Enol Viticult 16(3):144–158
Srivastav A, Ganjewala D, Singhal RK, Rajput VD, Minkina T, Voloshina M, Shrivastava M (2021) Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10(12):2556. https://doi.org/10.3390/plants10122556
Subbaiah LV, Prasad TNV, Krishna TG, Sudhakar P, Reddy BR, Pradeep T (2016) Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J Agric Food Chem 64(19):3778–3788. https://doi.org/10.1021/acs.jafc.6b00838
Sultana S, Naser H, Shil NC, Akhter S, Begum RA (2016) Effect of foliar application of zinc on yield of wheat grown by avoiding irrigation at different growth stages. Bangladesh J Agric Res 41(2):323–334. https://doi.org/10.23910/IJBSM/2016.7.5.1645b
Tabrez S, Khan AU, Hoque M, Suhail M, Khan MI, Zughaibi TA (2022) Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer. Nanotechnol Rev 11(1):2714–2725. https://doi.org/10.1515/ntrev-2022-0154
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. https://doi.org/10.1016/j.plaphy.2012.01.006
Tymoszuk A, Wojnarowicz J (2020) Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 13(12):2784. https://doi.org/10.3390/ma13122784
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69. https://doi.org/10.1016/j.plaphy.2016.08.022
Wang XP, Li QQ, Pei ZM, Wang SC (2018) Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol Plant 62(4):801–808. https://doi.org/10.1007/s10535-018-0813-4
Wang X, Yang X, Chen S, Li Q, Wang W, Hou C, Wang S (2016) Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plan Sci 6:1243. https://doi.org/10.3389/fpls.2015.01243 (eCollection 2015)
Wang Y, Meng Y, Ma Y, Liu L, Wu D, Shu X, Lai Q (2021) Combination of high Zn density and low phytic acid for improving Zn bioavailability in Rice (Oryza stavia L.) grain. Rice 14(1):1–12. https://doi.org/10.1186/s12284-021-00465-0
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55(396):353–364. https://doi.org/10.1093/jxb/erh064
Wu S, Hu C, Tan Q, Li L, Shi K, Zheng Y, Sun X (2015) Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium hirsutum). Acta Physiol Plant 37(8):1–9. https://doi.org/10.1007/s11738-015-1919-3
Xu Z, Rothstein SJ (2018) ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav 13(3):e1451708. https://doi.org/10.1080/15592324.2018.1451708
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535. https://doi.org/10.3389/fpls.2016.00535
Zhang W, Ebbs SD, Musante C, White JC, Gao C, Ma X (2015) Uptake and accumulation of bulk and nanosized cerium oxide particles and ionic cerium by radish (Raphanus sativus L.). J Agric Food Chem 63(2):382–390. https://doi.org/10.1021/jf5052442
Zhao FJ, McGrath SP (2009) Biofortification and phytoremediation. Curr Opin Plant Biol 12(3):373–380. https://doi.org/10.3389/fpls.2015.00136
Acknowledgements
The financial support was provided by Alzahra University.
Author information
Authors and Affiliations
Contributions
Mona Sorahinobar and Zahra Nazem Bokee designed experiments with the assistance of Ali Mehdinia, Toba Deldari carried out experiments, Mona Sorahinobar analyzed experimental results and data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Pearson correlation analysis of all assayed variables in mungean seedlings germinated and grown on MS media supplemented with 0, 10, 20, 40, 80, and 160 ppm ZnO NPs. (JPG 4.13 MB)
Supplementary Fig. 2
Growth biomarkers of mungbean seedlings grown on MS media containing 0, 10, 20, 40, 80 and 160 ppm zinc oxide nanaoparticles. Different letters indicate means that are significantly different at P ≤ 0.05. (JPG 300 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sorahinobar, M., Deldari, T., Nazem Bokaeei, Z. et al. Effect of zinc nanoparticles on the growth and biofortification capability of mungbean (Vigna radiata) seedlings. Biologia 78, 951–960 (2023). https://doi.org/10.1007/s11756-022-01269-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01269-3