Abstract
Schistosoma mansoni is the main factor of human schistosomiasis which is responsible for high rates of mortality. Recently, the use of alternative biological control agents has gained importance in disease control because the intensive use of molluscicides is very harmful to human health and poses risks to the environment. In the present work, the potential effect of two freshwater algae, Amphora coffeaeformis and Scenedesmus obtusus, on the immune response of Biomphalaria alexandrina snails against infection with S. mansoni was investigated. Two different concentrations 1 and 2 g L− 1 from each dried algal material were tested on snails before exposure to miracidial infection by one day. The use of Amphora coffeaeformis has a greater immunostimulatory effect than Scendesmus obtusus at a low concentration of 1.0 g L− 1. The tested algae affected the snail’s hemocytes and its immune response to S. mansoni as evidenced by a significant decrease in infection rate and cercariae production. In addition, increasing in total hemocyte count, the formation of vacuoles, the appearance of several pseudopodia, and the formation of coarse granules in hemocytes of infected snails treated with A. coffeaeformis. Intense tissue reactions were also observed. In conclusion, it was confirmed that these algae can be used as an immunostimulant in the prevention and control of S. mansoni.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is highly prevalent and second disease after malaria that affects millions of humans around the world, especially in developing countries (Kiros et al. 2014; Rees et al. 2019). Worldwide, Schistosoma mansoni (Sambon, 1907) infects approximately 240 million people every year (WHO 2014). Biomphalaria alexandrina (Ehrenberg, 1831) snail is an aquatic gastropod mollusk (family Planorbidae) and the intermediate host of S. mansoni (Faro et al. 2013). To complete the life cycle of Schistosoma, mature eggs are deposited in freshwater, which then hatches into miracidia that invade the snail, develop, multiply, and eventually become the mother sporocyst and stimulate the snail’s defense system. At the same time, the parasite attempts to escape the internal defenses of the snail host to continue its life cycle. Two weeks after penetration, the sporocysts reproduce multiple asexually, and finally, they migrate to the reproductive organs and hepatopancreas (digestive glands). After 30 days of its penetration, cercariae are shedded into the water to infect the final host (Nacif-Pimenta et al. 2012; Nelwan 2019).
Gastropods have an effective internal defense system (IDS) that differs from the immune system of vertebrates. This system comprises both cellular and humoral elements that act together to combat the infection (Bayne 2009; Saad et al. 2014, 2017; Coustau et al. 2015; Le Clec’h et al. 2016). The hemolymph of freshwater snails contains hemoproteins and different types of blood cells called hemocytes. There are different subpopulations and morphological variability of hemocytes, which play an important role in the recognition and attack of foreign bodies through different processes such as encapsulation, phagocytosis, and releasing some cytotoxic mediators (Bayne 1990; Miller et al. 2001). Previous studies reported that the immune mechanism that influences compatibility between S. mansoni and Biomphlaria snails depends primarily on the immune cells of snails (hemocytes). Also, the fibrinogen-related proteins and humoral immune effectors are concerned with the anti-schistosome immune response (Pila et al. 2017).
Microalgae exhibit different biological activities in the presence of a variety of phytoconstituents that are created by metabolic enzymes. These metabolites are effective alternatives to antibiotics especially to combat the disease (Selvendran 2013; Ayoub et al. 2019). Several investigators enhanced the role of algae as immunostimulants. They ensured that some algae like Amphora species and Chlorella species can increase the resistance of some animals like fish and mice (Morris et al. 2007; Ayoub et al. 2019). The enzymatic proteins of the green alga Chlorella vulgaris stimulate the defense activity of mammals by enhancing mechanisms of innate and specific immune responses (Morris et al. 2007). Algae have many advantages, the most important of which is that they are an important source of food, their easy cultivation gives them importance in immunomodulating studies. Amphora coffeaeformis (Agardh) Kutzing, 1844 is one of the most abundant species in alkaline fresh, brackish, and marine water (Bhosleac et al. 1993). It contains high amount of photosynthetic pigments such as chlorophyll, carotenoids with high levels, and also a series of biologically active substances with antioxidant, antiobesity, antimicrobial, and other properties used in many medical applications (Gories et al. 2012; El-Sayed et al. 2018). Scenedesmus obtusus is one of the most common freshwater algae species, it has beneficial aspects, promoting enhanced immune response, good weight control, improved fertility, and a lustrous coat (Pulz and Gross 2004). The microalgae including A. coffeaeformis and S. obtusus are used in bioremediation for detoxification and to getting rid of environmental pollutants (Sayed et al. 2019). For these reasons, in recent years, the utilization of algae in pharmaceutical and medical applications has attracted global attention (El-Sayed et al. 2018; Ke Ma et al. 2020).
The aim of the current study is the assessment of A. coffeaeformis and S. obtusus as immunostimulant agents on B. alexandrina snails against Schistosoma infection. This will be achieved by reporting and understanding the hematological responses and tissue interaction of treated snails with different concentrations of algae against infection with S. mansoni.
Materials and methods
Snails and Schistosoma mansoni infection
Biompholaria alexandrina snails (3-6 mm in diameter) were obtained and reared in the Medical Malacology Department, Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt. They were put in plastic aquaria (16 × 23 × 9 cm) containing dechlorinated tap water (10 snails/L) at a temperature of 25 ± 1 °C. They were provided with dried lettuce leaves for feeding. Schistosoma mansoni strain that is sympatric with B. alexandrina strain was used and applied by the Schistosomiasis Biological Supply Center (SBSC) (TBRI, Giza) in this study. Eggs were collected from the livers of twelve CD1 mice livers infected 5–8 weeks earlier with 300 S. mansoni cercariae (El-Sheikha et al. 2008). About 200 ml of 0.9% NaCl were added to the minced liver, the suspension was homogenized using warring blender for 5–10 s at low speed. The homogenate was sieved using a tiered column of sieves of mesh opening (420 μm, 177 μm, 105 μm and 45 μm). The eggs were collected and rinsed with tap water. The eggs were transferred into a small petri dish and maintained under ceiling illumination for about 5–7 min to stimulate miracidial hatching.
Experimental material
Two freshwater algae Amphora coffeaeformis (C. Agardh) Kützing, 1844 and Scendesmus obtusus Meyen, 1829, were isolated from drainage water of Suez province by workers of the Algal biotechnology unit (National Research Centre, Dokki, Giza, Egypt) during summer of 2020. The collected algae were then dried by the method used by El-Sayed et al. (2001).
Exposure of snails to A. coffeaeformis and S. obtusus algae and S. mansoni miracidia
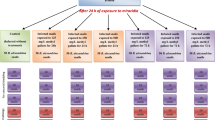
A number of 300 snails were divided into five groups of six replicates (3 replicates for hematological investigation and 3 ones for counting cercaria). Group 1 represented untreated infected snails (control). Groups 2 and 3 snails were treated with 1.0 g L− 1 and 2.0 g L− 1of A. coffeaeformis, respectively, for one day and then exposed to miracidia. Groups 4, and 5 were treated with 1.0 g L− 1 and 2.0 g L− 1 of S. obtusus respectively for one day and then exposed to miracidia. Each snail was individually exposed to 8–10 S. mansoni miracidia with 2 ml dechlorinated tap water for 24 h (Anderson et al. 1982). Then snails were transferred to clean aquaria and kept under the same laboratory conditions.
Three weeks post-infection (WPI), the surviving snails were individually examined for cercaria after three h of exposure to light. Positive snails were transferred to new clean aquaria and maintained in dark under laboratory conditions. Cercariae were counted under a stereomicroscope in a few drops of iodine solution. The survival and infection rates were calculated according to Youssef et al. (1998).
Hemolymph collection
Collection of hemolymph from all snail groups at 1, 2, and 3 WPI with S. mansoni miracidia was carried out. Shells of snails were cleaned with 70% alcohol and dried. The hemolymph was collected from randomly selected 5–10 snails from each group by a cardiac puncture using a 21-gauge needle, 20 µl of hemolymph was obtained from each snail according to Martins-Souza et al. (2006).
Total hemocytes count
The freshly collected hemolymph was diluted in a leucocyte count solution 1:20 ratio. The Bürker-Turk hemocytometer was used for counting the number of hemocytes in each group.
Differential hemocytes count
Hemocyte monolayers were prepared by placing of 10 µl of hemolymph on a glass slide for 15 min in a moist chamber for adherent hemocytes then fixed in 100% methanol for 5 min and stained with Giemsa stain (Aldrich) (10%) in buffered distilled water (0.021 M Na2HPO4/0.015 M KH2PO4) pH 7.2 for 20 min. Slides were then washed with buffered distilled water and subsequently examined under a light microscope (Barracco et al. 1993) to calculate the percentage of hemocyte population in the group.
Histological study
The infected snails that were previously exposed to different concentrations of A. coffeaeformis and S. obtusus were used for histopathological investigations. Three to five snails were selected randomly and dissected after six weeks of recovery from algal exposure. They were relaxed with menthol crystals (approximately 2 × 10− 4 M). Each snail was then carefully crushed between two microscope slides, and the broken shell was removed from the body. The columellar muscle was separated from the shell and fixed in Bouin’s fixative for at least 24 h and then placed in gradually increasing concentrations of ethanol, cleared with xylol, then embedded in paraffin, and finally sectioned at 5 μm. Sections were stained with hematoxylin and eosin stain and dried, and then, the slides were examined microscopically (Borges et al. 1998) for the histological condition of larval trematodes and the effect of algae to stimulate tissue reactions of treated snails against S. mansoni.
Statistical analysis
The Chi-square test was used to determine the significant differences in survival rate, infection rate, and percentage of hemocytes between the control and the treated groups and between every two similar treatments. Total hemocyte count and the number of shedded cercariae were evaluated using one-way ANOVA at (p < 0.05). The obtained data were analyzed using SPSS 0.19 computer program.
Results
Effect of dried algal material of Amphora coffeaeformis and Scendesmus obtusus on the susceptibility of B. alexandrina snails to infection with S. mansoni miracidia
Figure 1 shows that there is no significant difference in survival rate between all experimental groups. Generally, the infected snails previously exposed to different concentrations of both algae have a significant decrease in their infection rate compared to the control group (p < 0.05). In the snails that exposed to 1.0 g L− 1of both algae A. coffeaeformis and S. obtusus (group 2 and 4), the infection rate decreased significantly than 2 g L− 1 of the same algae. In addition, the infection rate decreased significantly more in the infected snails previously treated with A. coffeaeformis than in those treated with S. obtusus (Fig. 2).
Percentage of infection in B. alexandrina snails with S. mansoni miracidia in control and exposed groups to algae A. coffeaeformis and S. obtusus. (The values in bars marked with the same letters are not significantly different while those marked with different letter(s) are significantly different (LSD at p ≤ 0.05 according to Duncan’s multiple range test)
Also, over four weeks, the mean number of cercariae in all treated snails was significantly lower than that in the control group (p < 0.05). As shown in Fig. 3 the most effective concentration of both algae that suppressed snails to produce cercaria was 1.0 g L− 1 with a significant difference between them (p < 0.05).
Mean number of cercaria per snail in control and treated groups with algae A. coffeaeformis and S. obtusus. The values in bars marked with the same letters are not significantly different while those marked with different letter(s) are significantly different (LSD at p ≤ 0.05 according to Duncan’s multiple range test)
Effect of dried algal material of A. coffeaeformis and S. obtusus on morphology and content of B. alexandrina hemocytes
Light microscopic examination of the normal hemolymph of B. alexandrina revealed three distinct cell types classified according to their shape and granular content. These cells are hyalinocytes, amoebocytes, and granulocytes. Granulocytes contain abundant dense granules in their cytoplasm. Hyalinocytes have transparent cytoplasm while amoebocytes have distinct pseudopodia (Fig. 4). B. alexandrina hemocytes of infected snails at 2 WPI to S. mansoni miracidia showed activation of some cells by the appearance of vacuoles in the cytoplasm, the formation of some pseudopodia and thick cell membranes (Fig. 5). In snails that treated with 1 g L− 1 A. coffeaeformis algae and then exposed to S. mansoni miracidia at 2 WPI, hemocytes showed greater activation by the formation of more pseudopodia, several vacuoles and many coarse granules (Fig. 6). The vacuoles that near to surface of the cell became ready to release their content out of the cell. The hemocytes of B. alexandrina were less activated than those with A. coffeaeformis after exposure to 1 g L− 1 S. obtusus and subsequent exposure to S. mansoni miracidia for two weeks.
Total hemocytes count in unexposed and exposed snails to A. coffeaeformis and S. obtusus
The infected snails with miracidia previously exposed to 1.0 and 2.0 g L− 1 of algal material A. coffeaeformis and S. obtusus caused a gradual increase in hemocyte count compared with the unexposed snails. The increase in circulating hemocytes became highly significant at 2 WPI compared with the control (group 1) then a decrease occurred. The increase in hemocyte count in snails treated with 1.0 g L− 1 of A. coffeaeformis and 1 g L− 1 of S. obtusus was significantly higher than in those treated with 2 g L− 1 of two algae. The hemocyte count in snails treated with A. coffeaeformis before infection with miracidia was significantly higher than in snails treated with S. obtusus (Table 1).
Differential hemocytes count
Three types of hemocytes were recorded in the present study; granulocytes, amoebocytes, and hyalinocytes with different percentages after exposure to miracidia. Table 2 showed a significant increase in the percentage of granulocytes and amoebocytes in all snail groups compared to hyalinocytes. The percentage of hyalinocytes in all treated snails decreased compared to the control group.
Effect of algae on tissue reactions of snails against S. mansoni
Six weeks post-exposure in normally infected snails, numerous developing daughter sporocysts and developmental stages of cercariae were observed within several tissues and organs with an increase in size and number of germ cells inside them (Fig. 7a, b). The sporocysts appeared as space-occupying lesions. No encapsulation was observed in infected snails. In contrast, the infected snails previously exposed to A. coffeaeformis algae, most sporocysts were dead at 6 WPE, many necrotic sporocysts and a number of residual ones were observed in cephalopodal tissue (Fig. 8a, b). It was observed that the snails that exposed to algae revealed hemocyte proliferation with focal thickening of the stroma and granulomatous reaction (Fig. 8c). They also showed degradation of the sporocysts especially in the head-foot region and in tentacles because of retarded infection dynamics. Moreover, numerous hemocytes were found near these sporocysts (Fig. 8d, e). Dead sporocysts appeared as eosinophilic masses surrounded by multiple layers of hemocytes (Fig. 8f). Only a few sporocysts and a few developmental stages of cercariae were able to migrate around the digestive gland. Numerous large granulomata were observed around the remnant of sporocysts. These granulomata consist of hemocytes and fibers surrounding the dead sporocysts (Fig. 8g). Also, germinal cells were presented inside sporocysts that lacked nucleoli (Fig. 8h). A cellular reaction formed around some viable immature cercariae. The stroma of the inflammatory foci and an increased amount of amorphous and fibrillar components of the extracellular matrix were noted (Fig. 8i).
Histological sections of infected snails at 6 WPI previously exposed to A. coffeiformis. a, b Number of necrotic sporocysts (S) and a number of residual sporocysts (arrows) were observed. c Extensive hemocyte proliferation and granulomatous reaction are seen around a few disintegrating parasites in the head foot region. d Destroyed sporocysts in the tentacles of snails surrounded by several layers of hemocytes (arrows). e Destroyed sporocysts (S) in the head- foot, numerous hemocytes (arrows) were settled in the proximity of the sporocysts. The size of the vacant space was wider than in normal sporocyst (arrows head). f The sporocysts (s) is destroyed and surrounded by several layers of flattened hemocytes (arrows). g A granuloma around a dead sporocyst consisting of hemocytes (arrowhead) and fibers (arrow) between digestive glands. -h- Destroyed immature cercariae and sporocysts which contained germinal cells that lacked nucleoli and were surrounded by numerous hemocytes (arrow). i The cercariae (arrow) encircled by a strong stromal reaction between digestive tubules
On the other hand, a few sporocysts had died in infected snails previously exposed to S. obtusus. Small granulomata were formed around the remnant of sporocysts in cephalopodal tissues. Some observed sporocysts exhibited morphological damage and were surrounded by numerous hemocytes (Fig. 9a). Numerous fibrous cells of snails were seen surrounding these encapsulated and destroyed sporocysts, which had lost their normal structure (Fig. 9b). Hemocytes were settled near the destroyed sporocysts in the head foot region and the tentacles due to retarded infection and a small concentration of fibrous cells around the destroyed sporocyst forming a thin layer was also observed (Fig. 9c). A moderate number of sporocysts could be seen migrating around the digestive gland with a moderate number of dividing germ cells inside it and developmental stages of cercariae (Fig. 9d). This cellular response is not as intense as in infected snails previously exposed to A. coffeaeformis.
Histological sections of B. alexandrina infected snails at 6 WPI previously exposed to S. obtusus. a Destroyed sporocyst (s) surrounded by several layers of hemocytes (arrow) in cephalopodal tissue. b The sporocyst (s) is damaged and formation of a thick layer around the extremities of the parasite (arrows) in head foot. c The sporocyst is completely destroyed, a small concentration of fibrous cells (arrows head) around it forming a thin layer and numerous hemocytes (arrow) were settled in the proximity of the sporocysts in tentacles. d Sporocysts (s) between digestive glands contained few germinal cells and the size of the vacant space was wider than normal
Histological examination revealed that there was a large difference between normally infected snails and previously treated snails with algae. The differences were evident in a low number of cercariae in the tissue, a high number of degenerating sporocysts, and intense cellular response in the treated snails compared to the normally infected snails.
Discussion
Schistosomiasis remains a serious public health problem around the world particularly in Africa, South America, the Middle East, and the Caribbean. The use of molluscicides to control transmission is not recent (Augusto and Mello-Silva 2018). Recently, there has been an increased focus on immunostimulants to improve the host defense response and increase resistance to various diseases. The use of alternative biological control agents targeting the intermediate host Biomphalaria snail has gained importance in disease management because the intensive use of molluscicides is very harmful to human health and poses risks to the environment. So, scientists are increasingly turning their attention to algae as ingredient factories, particularly the nutritional components. Microalgae including Amphora coffeaeformis and Scendesmus obtusus have been considered to be very useful and applicable organisms in many fields such as medicine, food, dietary supplement, and wastewater treatment (Torres 2016; Ke Ma et al. 2020). So, the current work investigated the role of two species of freshwater algae A. coffeaeformis and S. obtusus as natural immunostimulant materials on B. alexandrina snails. The current data showed a significant reduction in the susceptibility of snails to S. mansoni infection and the number of shed cercaria in all treated groups as compared to the control one. The low concentration is the most effective. A similar reduction in cercarial production rate was obtained by El-Sayed et al. (2011) who observed a significant reduction in the infection rate of B. glabrata and B. alexandrina that maintained in LC10 of an aqueous solution of cryptostegia grandiflora prior to exposure to miracidia. The current study agrees with the obtained results by Soliman et al. (2017) who stated that snails exposed to the lowest concentration of β- glucan led to a decrease in their infection rate. Also, Ayoub et al. (2019) reported that A. coffeaeformis improved the serum protein, and the lysozyme and increased disease resistance of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) fish.
The first line of defense in mollusks is controlled by circulating hemocytes in the hemolymph and their ability to respond strongly to stimuli (El Sayed et al. 2011; Mossalem et al. 2017). The present study showed that the hemolymph of the snails is composed of three types of hemocytes: granulocytes, amoebocytes, and hyalinocytes which agrees with the results introduced by (El Sayed et al. 2011; Mossalem et al. 2017). The present study showed that the hemolymph of the snails is composed of three types of hemocytes: granulocytes, amoebocytes, and hyalinocytes which agrees with the results introduced by Kamel et al. (2006); El Sayed et al. (2011) and Helal et al. (2014). Contrary to the present study, Mohamed (2011) indicated that B. alexandrina hemocytes can be classified according to cell shape and size into two types: small, round hyalinocytes and granular spreading. The present work showed that the exposure of snails to algae can change the profile of their hemocytes and influence the immune response to S. mansoni. The hematological study of hemolymph of infected B. alexandrina snails previously exposed to A. coffeaeformis showed several vacuoles within hemocytes, the appearance of many pseudopodia, and the formation of coarse granules in granulocytes. The hemocytes that contain coarse granules were occasionally detected and known to transport metabolic substances from the digestive glands or may be involved in the aggregation process (Nakayama et al. 1997; Kheder 2020). The present findings match those introduced by Ibrahim and Abdel-Tawab (2020) who estimated that snails treated with LC50 of algal extract (Cystosiera barbata) caused the formation of pseudopodia in hemocytes. Similarly, Mossalem and Mossa (2014) recorded the same results due to the treatment of Biomphalaria snails with rice bran extract before and after infection.
The present work showed a significant increase in the percentage of amoebocytes and granulocytes in all treated snails compared to the control group while, percentage of hyalinocytes decreased. It was declared that granulocytes are the main cells engaged in snail defense, as they are involved in parasite encapsulation (Loker et al. 1982). The current results are in accordance with Sparks’s results (1972) who stated the role of granulocytes and amoebocytes in eliminating living pathogens and engulfing them through enzymatic or oxidative degradation. Granulocytes and amoebocytes are the most abundant cells type in snail’s hemolymph that perform phagocytosis process and encapsulation reactions (Loker et al. 2004; Yoshino and Coustau 2011; Barçante et al. 2012). The increase in the number of granulocytes and amoebocytes in experimental groups was more than in the control group, which predisposes these snails to exhibit low susceptibility and significant reduction of cercarial production in the present study. In agreement with to present work, studies carried out on Biomphalaria tenagophila (Orbigny 1835) demonstrate that higher granulocytes count is related to resistance because of their role in encapsulation that leads to parasite removal (Pereira et al. 2006; Pila et al. 2017). It is known that hyalinocytes are small spherical cells unable to adhere to substrates. They were responsible primarily for wound repair, requiring aggregation at an injury site. On other hand, granulocytes are large amorphous cells with granules in the cytoplasm, which easily adhere to substrates and form pseudopods (Bezerra et al. 2003; Helal et al. 2014).
The current result showed that infected snails previously exposed to 1 and 2 g L− 1 of algal material A. coffeaeformis and S. obtusus caused a gradual increase in the number of hemocytes count in comparison with the unexposed snails. The increase in circulating hemocytes became highly significant at 2 WPI compared to control (group1) then a decrease occurred. The number of hemocytes was significantly higher in snails treated with A. coffeaeformis than in snails treated with S. obtusus. This may be due to that the cells originating within amoebocyte-producing organs flowing with the hemolymph to concentrate at the sites of infection. In this way, a higher concentration of hemocytes was observed in the hemolymph of treated snails (Souza Sdos and Andrade, 2012). The alga A. coffeaeformis was able to stimulate this organ to produce more hemocytes than S. obtusus. Moreover, maintaining a higher number of hemocytes plays an important role in the immune response of snails, as it is the driving force in a successful immune response. The present observations are in accordance with the results stated by Maftuch et al. (2012) in which marine algae (Gracilaria verrucosa) enhanced some innate immune responses in shrimp (Penaeus monodon) and increased its resistance against Vibrio harveyi infection. In the same way, Mossalem et al. (2017) recorded a significant increase in the hemocytes after exposure of infected B. alexandrina to Punica granatum peels extract. They attributed that to enhancing defense mechanisms against parasites produced by hemocytes. Saad et al. (2017) demonstrated that increased numbers of hemocytes in infected Bulinus truncatus (Audouin, 1827) snails indicated low susceptibility to S. haematobium. These results are consistent with the present work, as exposure of snails to algae stimulated their immune response, resulting in a decrease in infection rate and the number of cercariae. Natural wild algae can be classified as immunostimulants due to their ability to stimulate a non-specific immune response and enhance the growth process (Ke Ma et al. 2020).
The histological study revealed that there were great differences between normally infected snails and previously treated infected snails with algae mainly noticed as a proliferation of hemocytes, death of sporocysts in tissues with the presence of granulomatous structure, and retarded infection dynamics. In the same way, Mansour et al. (2021) reported that the histopathological responses of infected Biomphlaria snails exposed to methyl gallate as an immunostimulant showed few cercariae and strong hemocytic reaction. The algae are able to influence S. mansoni in snail tissues, reducing the cercariae shedding in water and changing S. mansoni life cycle. These results are in agreement with the results by Lewis et al. (1993) who stated that the ability of infected snails to shed cercariae is very correlated with histopathological studies. In B. glabrata highly susceptible snails, sporocysts and cercariae are found in great numbers, and absence of any snail tissue reaction. On the contrary, less susceptible snails, shedding few cercariae exhibit marked diffuse hemocyte proliferation in many organs and tissues, resulting in the encapsulation of dead parasites. By observing tissue reactions of snails treated with A. coffeaeformis in the present study, the immune responses might be happened by one of two different mechanisms: one type of defense utilized direct miracidia destruction soon after their penetration. In these snails, the intense hemocyte proliferation with focal thickening of the stroma was present in the cephalopodal tissue at the site of the penetration of the miracidia. A focal hemocytic infiltration was observed in the cephalopodal tissue of the highly resistant B. tenagophila infected snail that was associated with rapid parasite destruction after penetration (Negrão-Corrêa et al. 2007). The second type of immune reaction demonstrated that the formation of granuloma around the digestive gland and encapsulating was formed around a collection of viable cercariae found in the treated infected tissues. Borges et al. (1998) considered these reactions as a delayed development of resistance that occurred after sporocysts spread in the snail tissues and this represents an alternative type of snail internal defense mechanism against S. mansoni. Mansour et al. (2021) reported that exposure of infected B. alexandrina snails to low concentration of methyl gallate increased the tissue response in the digestive gland and cephalopdal region represented by many hemocytes around the sporocysts and cercariae trying to destroy the parasite. Amphora coffeaeformis stimulates the synthesis of hemocytes containing coarse granules that can activate the hemocyte aggregation process and granuloma formation. The tissue reactions in infected snails treated with S. obtusus showed direct miracidial destruction soon after their penetration as numerous dense layers of host fibrous cells were seen surrounding these damaged parasites and all characteristic structures were destroyed in cephalopodal tissues. In the same way, exposure of infected B. alexandrina snails to ¼ LC50 of inorganic fertilizers enhanced the tissue response by aggregation of hemocytes around mother sporocysts trying to attack them in the cephalopodal tissues (Hussein et al. 2016).
Conclusion
The snail’s immune response is an important determinant of Schistosoma compatibility. The tested algae have a schistostatic effect and can be used as indirect therapy because they can be put in the water to influence the life cycle in host and it is a natural product. Also, there is a modern trend to use these microalgae as immunostimulants in the prevention and control of infectious diseases such as schistosomiasis. Amphora coffeaeformis is more effective immunostimulant than Scendesmus obtusus. This was reflected in a reduction of B. alexandrina susceptibility to S. mansoni, hematological and tissue reaction of treated snails.
References
Anderson RM, Mercer JG, Wilson RA, Carter NP (1982) Transmission of Schistosoma mansoni from man to snail: experimental studies of miracidial survival and infectivity in relation to larval age, water temperature, host size and host age. Parasitol 85:339–360. https://doi.org/10.1017/s0031182000055323
Augusto RC, Mello-Silva CC (2018) Phytochemical molluscicides and schistosomiasis: What we know and what we still need to learn. Vet Sci 5(94):1–9. https://doi.org/10.3390/vetsci5040094
Ayoub HF, Abdelghany MF, El-Sayed AB (2019) Effects of diatoms Amphora coffeaeformis on growth parameters, nonspecific immunity and protection of the Nile tilapia (Oreochromis niloticus) to Aeromonas hydrophila infection. Egypt J Aquat Biol Fish 23(1):413–426
Barçante TA, Barçante JMP, Fujiwara RT, Lima WS (2012) Analysis of circulating haemocytes from Biomphalaria glabrata following Angiostrongylus vasorum infection using flow cytometry. J Parasitol Res 1–6. https://doi.org/10.1155/2012/314723
Barracco MA, Steil AA, Gargioni R (1993) Morphological characterization of the hemocytes of the pulmonate snail Biomphalaria tenagophila Mem Inst Oswaldo Cruz 88(1):73–83
Bayne CJ (1990) Phagocytosis and non-self recognition in invertebrates. Bioscience 40:723–731
Bayne CJ (2009) Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: an assessment. Mol Biochem Parasitol 165(1):8–18. https://doi.org/10.1016/j.molbiopara.2009.01.005
Bhosleac NB, Evansad LV, Edyveanb RGJ (1993) Carbohydrate production by Amphora coffeaeformis, a marine fouling diatom. Biofouling 7(1):81–91
Bezerra FSM, Nogueira-Machado JA, Chaves MM, Correa RF, Coelho PMZ (2003) Effect of gamma radiation on the activity of hemocytes and the course of Schistosoma mansoni in resistant Biomphalaria glabrata. Mem. Inst Oswaldo Cruz 98:73–75. https://doi.org/10.1590/S0074-02762003000100010
Borges CM, Souza CP, Andrade ZA (1998) Histopathological features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Mem Inst Oswaldo Cruz 93:117–121. https://doi.org/10.1590/S0074-02761998000700016
Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G (2015) Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish Shellfish Immunol 46(1):5–16. https://doi.org/10.1016/j.fsi.2015.01.036
El-Sayed AB, Abdalla FE, Abdel- Maguid AA (2001) Use of some commercial fertilizer compounds for Scenedesmus cultivation. Egypt J Phycology 2:9–16
El-Sayed AB, Aboulthana WM, El-Feky AM, Ibrahim NE, Seif MM (2018) Bio and Phyto-chemical effect of Amphora coffeaeformis extract against hepatic injury induced by paracetamol in rats. Mol Biol Rep 45(6):2007–2023. https://doi.org/10.1007/s11033-018-4356-8
El Sayed KA, Mahmoud MB, Mossalem HS (2011) Cryptostegia grandiflora affecting compatibility of Biomphalaria alexandrina and Biomphalaria galabrata to infection with Schistosoma mansoni with emphasis on some hematological effects. Aust J Basic Appl Sci 5(12):3357–3365
El-Sheikha HM, Hussein HS, Rahbar MH (2008) Clinico-pathological effects of Schistosoma mansoni infection associated with simultaneous exposure to malathion in Swiss outbred albino mice. Acta Trop 108:11–19. https://doi.org/10.1016/j.actatropica.2008.07.008
Faro MJ, Perazzini M, Correa LD, Mello-Silva CC, Pinheiro J, Mota EM, Souza S, Andrade Z, Junior AM (2013) Biological, biochemical and histopathological features related to parasitic castration of Biomphalaria glabrata infected by Schistosoma mansoni. Exp Parasitol 134:228–234. https://doi.org/10.1016/j.exppara.2013.03.020
Goiris K, Muylaert K, Fraeye I, Foubert I, De Brabanter J, De Cooman L (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24(6):1477–1486
Helal EG, El-Dafrawy SM, Mohamed AH, Abou-El-Nour BM, Ibrahim S (2014) Ultrastructural study on Biomphalaria alexandrina hemocytes infected with Schistosoma mansoni in Egypt and its correlation with nitric oxide level. J Egypt Soc Parasitol 44:113–124. https://doi.org/10.12816/0006450
Hussein RM, Marie MAS, El-Deeb FAA, Hasheesh W, Sayed SSM (2016) Effects of three inorganic fertilizers on the biology and histopathology of infected Biomphalaria alexandrina snails. Res J Pharm Biol Chem Sci 7:2564–2574
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schistosoma mansoni. Biologia 75:1945–1954. https://doi.org/10.2478/s11756-020-00457-3
Kamel AG, Refaat SM, El-Dafrawy SM, Mohamed AH, Mossalem SHH (2006) The effect of Schistosoma mansoni infection on Biomphalaria alexandina haematocytes at ultrastructure level. In: Proc 4th Int Cong Biol Sci (Zool.), pp 219–226
Ke Ma, Bao Q, Wu Y, Chen S, Zhao S, Wu H, Fan J (2020) Evaluation of microalgae as immunostimulants and recombinant vaccines for diseases prevention and control in aquaculture. Front Bioeng Biotechnol 8:1–12. https://doi.org/10.3389/fbioe.2020.590431
Kheder SI (2020) Morphology and kinetics of susceptible and resistant Biomphalaria alexandrina hemocytes during the first week of exposure to Schistosoma mansoni miracidia. Parasitol United J 13(3):179–189. https://doi.org/10.21608/puj.2020.47046.1089
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:1–7
Lewis FA, Richards CS, Knight M, Cooper LA, Clark B (1993) Schistosoma mansoni: Analysis of unusual infection phenotype in the intermediate host snail Biomphalaria glabrata. Exp Parasitol 77:349–391. https://doi.org/10.1006/expr.1993.1092
Loker ES, Bayne CJ, Buckley PM, Kruse KT (1982) Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J Parasitol 68:84–94
Loker ES, Adema CM, Zhang SM, Kepler TB (2004) Invertebrate immune systems not homogeneous, not simple, not well understood. Immunol Rev 198:10–24. https://doi.org/10.1111/j.0105-2896.2004.0117.x
Le Clec’h W, Anderson TJC, Chevalier FD (2016) Characterization of hemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosom amansoni infection. Parasit Vectors 9(32):1–11. https://doi.org/10.1186/s13071-016-1319-6
Maftuch M, Toban H, Risjani Y (2012) Administration of marine algae (Gracilaria verrucosa) immunostimulant enhances some innate immune parameters in black tiger shrimp (Penaeus monodon Fabricus) against Vibrio harveyi infection. J Appl Sci Res 8(2):1052–1058
Martins-Souza RL, Pereira CA, Martins Filho OA, Coelho PMZ, Correa A (2006) Differential lectin labeling of circulating hemocyte from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni infection. Mem Inst Oswaldo Cruz 101:185–192. https://doi.org/10.1590/S0074-02762006000900029
Mansour SM, Sayed S, Abel- Wareth (2021) Effect of methyl gallate on immune response of Biomphalaria alexandrina (Ehrenberg, 1831) snails to infection with Schistosoma mansoni (Sambon, 1907). Parasitol Res 120:1011–1023. https://doi.org/10.1007/s00436-020-07037-z
Miller AN, Raghavan N, FitzGerald PC, Lewis FA, Knight M (2001) Differential gene expression in haemocytes of the snail Biomphalaria glabrata: effects of Schistosoma mansoni infection. Int J Parasitol 31:687–696. https://doi.org/10.1016/s0020-7519(01)00133-3
Mohamed AH (2011) Sublethal toxicity of Round up to immunological and molecular aspects of Biomphalaria alexandrina to Schistosoma mansoni infection. Ecotox Environ Safe 74:754–780. https://doi.org/10.1016/j.ecoenv.2010.10.037
Morris Hj, Carrillo O, Almarales A, Bermude RC, Lebeque Y, Fontaine R, Lourado G, Beltran Y (2007) Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzyme Microb Technol 40:456–460. https://doi.org/10.1016/j.enzmictec.2006.07.021
Mossalem HS, Mossa ATH (2014) Effect of rice bran extract on immunological and physiological parameters of Biomphalaria alexandrina snails infected with Schistosoma mansoni. Afr J Pharm Pharmacol 8(22):621–628. https://doi.org/10.5897/AJPP2013.3965
Mossalem HS, Ghareeb MA, Refahy LA, Mohamed AS, Habib MR (2017) Gas chromatography-mass spectrometry analysis and antioxidant activity of Punica granatum L. peels and its role as immunostimulant against Schistosoma mansoni infection in Biomphalaria alexandrina. Asian J Pharm Clin Res 10(1):252–258. https://doi.org/10.22159/ajpcr.2017.v10i1.15107
Nacif-Pimenta R, Mattos ACA, Orfanó AS, Barbosa L, Pimenta PFP, Coelho PMZ (2012) Schistosoma mansoni in susceptible and resistant snail strains Biomphalaria tenagophila: In vivo tissue response and in vitro hemocyte interactions. PLoS ONE 7(9):1–12. https://doi.org/10.1371/journal.pone.0045637
Nakayama K, Nomoto AM, Nishijima M, Maruyama T (1997) Morphological and functional characterization of hemocytes in the Giant Clam Tridacna crocea. J Invertebr Pathol 69:105–111
Negrão-Corrêa D, Pereira CAJ, Rosa FM, Martins-Souza RL, Andrade ZA, Coelho PMZ (2007) Molluscan response to parasite, Biomphalaria and Schistosoma mansoni interaction. Inverteb Surv J 4:101–111
Nelwan ML (2019) Schistomiasis: life cycle, diagnosis and control. Curr Ther Res 91:5–9. https://doi.org/10.1016/j.curtheres.2019.06.001
Orbigny A (1835) Synopsis terrestrium et fluviatilium molluscoum, in suo per Americam meridionalem itinere collectorum. Mag Zool 5(62):1–44
Pereira CA, Martins-Souza RL, Coelho PMZ, Lima WS, Negrão-Corrêa D (2006) Effect of Angiostrongylus vasorum infection on Biomphalaria tenagophila susceptibility to Schistosoma mansoni. Acta Trop 98:224–233. https://doi.org/10.1016/j.actatropica.2006.05.002
Pila EA, LiH, Hambrook JR, Wu X, Hanington PC (2017) Schistosomiasis from a snail’s perspective: Advances in snail immunity. Trends Parasitol 33(11):845–857. https://doi.org/10.1016/j.pt.2017.07.006
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–664. https://doi.org/10.1007/s00253-004-1647-x
Rees CA, Hotez PJ, Monuteaux MC, Niescierenko M, Bourgeois FT (2019) Neglected tropical diseases in children: An assessment of gaps in research prioritization. PLoS Negl Trop Dis 13(1):1–14. https://doi.org/10.1371/journal.pntd.0007111
Saad AH, Gawish FA, Abu El Einin H, Mansour SM (2014) Cytokines and mother sporocysts in susceptible and resistant Bulinus truncatus snails infected with Schistosoma haematobium. J Egypt Soc Parasitol 44(2):285–293. https://doi.org/10.12816/0006467
Saad AH, El Einin HA, Abdel-Gaber R, Ayoub M, Mansour SM (2017) Assessment of Bulinus truncatus immune response against Schistosoma haematobium infection by tissue reaction and hemocytes count. Res J Pharm Biol Chem Sci 8(3):1186–1196
Sayed AH, Kitamura D, Oda S, Kashiwada H, Mitani (2019) Cytotoxic and genotoxic effects of arsenic on erythrocytes of Oryzias latipes: bioremediation using Spirulina platensis. J Trace Elem Med Biol 55:82–88. https://doi.org/10.1016/j.jtemb.2019.06.007
Selvendran M (2013) Studies on antimicrobial compounds from selected marine phytoplanktons. Int J Pharm Biol Sci 4(2):876–888. https://doi.org/10.18000/ijabeg.10087
Soliman MG, El Sayed K, Abou Ouf NA, El Fekky FAEH, Gad RM (2017) Influence of immunostimulatory β-glucan on Biomphalaria alexandrina snails under laboratory and simulated field conditions. Eur J Biom Pharm Sci 4(12):41–50
Souza Sdos, Andrade ZA (2012) The significance of the amoebocyte-producing organ in Biomphalaria glabrata. Mem Inst Oswaldo Cruz 107(5):598–603. https://doi.org/10.1590/S0074-02762012000500005
Sparks AK (1972) Invertebrate pathology: Noncommunicable diseases, 1st edn. Academic, New York
Torres EM (2016) Microaalgae sorption of ten individual heavy metals and their effects on growth and lipid accumulation. Dissertations, Mechanical and Aerospace Engineering department, Utah State University
WHO (2014) Schistosomiasis. Fact sheet number 115
Yoshino TP, Coustau C (2011) Immunobiology of Biomphalaria-trematode interactions. In: Toledo R, Fried B (eds) Biomphalaria snails and larval trematodes. Springer, New York, pp 159–189
Youssef F, Ibrahim A, El- Bardicy SN (1998) Compatibility of Biomphalaria alexandrina, Biomphalaria glabrata and a hybride of both to seven strains of Schistosoma mansoni from Egypt. J Egypt Soc Parasitol 28:863–881
Acknowledgements
The authors acknowledge Theodor Bilharz Research Institute to support publication.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to this study, including material preparation, data collection, and analysis. The first draft of the manuscript was written by [Shereen Mahfouz Mansour] and [Rania Gamal Taha]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval was waived by the local Ethics Committee of Theodor Bilharz Research Institute.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansour, S.M., Taha, R.G. & Youssef, A.A. Assessment of Amphora coffeaeformis and Scenedesmus dimorphus algae as immunostimulant agents on Biomphlaria alexandrina snails against Schistosoma mansoni. Biologia 78, 737–748 (2023). https://doi.org/10.1007/s11756-022-01262-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01262-w