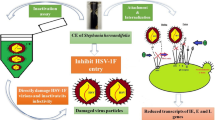
Abstract
Mushrooms produce various classes of secondary metabolites that could be used as antivirals in the future. The aim of this study was to determine the antiviral activity of methanolic extracts obtained from two edible mushrooms, Boletus bellinii (B. bellinii) and Boletus subtomentosus (B. subtomentosus), collected from the north forests of Tunisia, against Herpes Simplex Virus type 2 and Coxsackie Virus B type 3. In vitro micro-inhibition assays and cytotoxicity screening were performed on Vero cells. The tested Boletus methanolic extracts were found to be non-cytotoxic at high doses (50% cytotoxic concentration – CC50 > 1 mg/mL) and exhibited relevant viral inhibition with 50% inhibitory concentration, i.e., IC50 of 3.60 ± 0.66 µg/mL and 35.70 ± 7.42 µg/mL for B. bellinii, and 5.67 ± 1.02 µg/mL and 56.88 ± 9.56 µg/mL for B. subtomentosus, against HSV-2 and CVB-3, respectively. Interestingly, Boletus methanolic extracts showed high selectivity index (SI) values against both viruses, with the highest values against HSV-2 (SI > 800). Both viral strains were inhibited when treated with extracts during the early stages of virus replication. Inonotusin A was isolated and identified as the compound responsible for these activities. The latter is a novel antiviral agent that may have clinical utility or serve as a lead compound for further development. This study is the first attempt to investigate the antiviral activity of inonotusin A, isolated from the genus Boletus. The information from the present work should be a valuable reference for future studies on the antiviral activity of inonotusin A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction.
Viral infection is a global health problem affecting large populations all over the world. Virus elimination is complicated by the high prevalence of viral infections with no specific treatment and the constant appearance of new serotypes in virus groups (Pekosz and Glass 2008; Linnakoski et al. 2018). Currently, the best recognized examples of viral diseases are human immunodeficiency virus (HIV), herpes simplex virus, hepatitis, influenza and COVID-19 (Antonio et al. 2020). For thousands of years, natural products have served as traditional medicine and still provide the main source of biologically active molecules and they are potentially useful for drug development (Amzat and Razum 2018). One-third of all new molecular entities registered by the United States Food and Drug Administration (FDA) are based on natural products or their derivatives. Around one-half of these are derived from mammals, one-quarter from plants, and one-quarter from microbes (Patridge et al. 2015). Fungi represent a great source of bioactive molecules, which could potentially be used for novel drug discoveries (Linnakoski et al. 2018). Many studies have shown that a wide range of compounds with various biological activities isolated from mushrooms are of interest for medical applications due to their potential antitumor, immunostimulating, antibacterial, and antiviral effects (Lindequist et al. 2005; Santoyo et al. 2012). The bioactive mushroom products with antiviral properties are less extensively studied and characterized. However, some investigations have shown that bioactive compounds isolated from mushrooms offer a vast and unexplored diversity of chemical structures that can inhibit the virus infection by specifically targeting the viral enzymes, viral nucleic acid synthesis, or host cellular factors that the viruses use for their reproduction (Santoyo et al. 2012; Teplyakova and Kosogova 2015). On the other hand, direct antiviral effects have been mainly associated with the synthesis of phenolic secondary metabolites, whereas indirect antiviral effects have been linked to polysaccharides or other high-molecular weight molecules (Lindequist et al. 2005; Rincão et al. 2012).
Tunisian forests are known for the variety of their soils and climatic conditions. This variability results in a wide range of wild mushroom production and provides interesting opportunities for the discovery of novel natural antiviral compounds. Boletus species are among the most widespread wild mushrooms in North Tunisia’s forests (Ouali et al. 2018). The Boletales order include 300 species of Boletus whose spores are discharged from the surfaces of tubes beneath the mushroom cap (Money 2016). Carpophores of Boletus species have been frequently reported in conifer-dominated ecosystems. They are commonly found in pine forests, where they form mycorrhizal associations with a large variety of Pinaceae genera (Klofac 2013). Several species of the Boletales have been found to contain a diverse range of bioactive molecules with a high potential for therapeutic use, including phenolic secondary metabolites (Reis et al. 2011) and high-molecular-weight compounds (Zhang et al. 2018). However, few studies have been conducted on the antiviral activities of bioactive molecules isolated from Boletus species (Santoyo et al. 2012; Roy 2017; Linnakoski et al. 2018).
The appearance and dissemination of drug-resistant virus strains is a serious issue that strikes at the core of viral disease control. There is therefore an urgent necessity to develop new antiviral agents. The present study aimed to (i) assess the antiviral activity of two strains of Boletus against two viruses that are the causative agents of human pathologies, namely Herpes Simplex Virus type 2 (HSV-2) and Coxsackie Virus B type 3 (CVB-3) and (ii) identify the bioactive products and specify the mechanism of action of the characterized antiviral compound isolated from Boletus extracts within the different steps of the viral replication cycle.
The main novelties of this work are: (i) This is among the few works in the literature that investigate the antiviral activity of the genus Boletus; (ii) to the best of our knowledge, this is the first time that the antiviral compound identified as inonotusin A was isolated from Boletus species; (iii) this is the first study reports the antiviral activity of inonotusin A against CVB-3 and HSV-2.
Materials and methods
Chemical and reagents
Violet crystal, methylcellulose, methanol, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution were purchased from Sigma-Aldrich, ethanol and dimethyl sulfoxide (DMSO) from Scharlau, 96-well tissue culture plates and 35-mm dishes from Orange Scientific. Dulbecco’s Modified Eagle’s medium (DMEM), phosphate-buffered saline (PBS), antibiotics-antimycotic (100 X), trypsine-EDTA (10 X), and fetal bovine serum (FBS) were from Gibco BRL; Malt extract media for mushroom culture from Bio-Rad. Thin-layer chromatography (TLC) silica gel 60 F254 was bought from Silicycle.
Isolation and mushroom culture
The fruiting bodies of the B. bellinii and B. subtomentosus strains were collected from the North-Western Tunisia. In order to isolate mycelium in pure culture, small pieces of context (about 10 mm3) were aseptically drawn from the fruiting bodies and inoculated into malt extract agar (MEA) media with pH 6.0, in the dark at 25 °C for 15 days.
For the liquid culture, incubation was performed in 250 mL Erlenmeyer flasks containing 50 mL of 2% malt Extract Broth (MEB). Flasks with liquid medium were inoculated with three 8 mm diameter mycelial plugs cut from the Petri dishes using a sterile borer at the stage of actively growing mycelia. Mycelia were grown in static cultures for 14 days at 26 ± 2 °C.
Molecular identification of Boletus isolates
Ten days old mycelium grown in MEA was used for DNA extraction. Total genomic DNA from each sample was isolated using the FastDNA SPIN Kit (MP Biomedicals) following the manufacturer’s instructions. Nanodrop 2000 UV (Thermo Fisher, USA) was used to determine concentration and purity of total extracted DNA. PCR amplification was carried out by using ITS1 and ITS4 primers to amplify the internal transcribed spacer (ITS) region of the rDNA. The nucleotide sequence of ITS1 was 5’-TCCGTAGGTGAACCTGCGG-3’ and of ITS4 was 5’CAGGAGACTTGTACACGGTCCAG-3’ as described by White et al. (1990). PCR products were sequenced by Applied Biosystems 3130 Genetic Analyzer. The resulting sequences were deposited in the GenBank database. Sequence analyses were carried out by BLASTN similarity search at the website (http://www.ncbi.nlm.nih.gov/BLAST) of the National Center for Biotechnology Information (NCBI).
Preparation of fungal extracts
Extracellular crude extracts (ECEs) were obtained from liquid cultures of B. bellinii and B. subtomentosus. The fermentation products were filtered using Whatman no. 4 paper and centrifuged at 4,500 x g for 10 min. The existing proteins were extracted using Sevag reagent (chloroform: n-butyl alcohol, 4:1). To precipitate the crude extracts, a four-fold volume of methanol was added for 24 h at 4 ˚C. Following 30 min of centrifugation at 4,500 x g, the residue was then extracted three times with methanol. After dialysis, the combined extracts were evaporated at 40 °C under reduced pressure (rotary evaporator) to remove methanol and freeze-dried using a vacuum freeze dryer with primary drying at 60 mT vacuum and a shelf temperature set at ‑100 ˚C for 20 h. The freeze-dried extracts were reconstituted for experimental use in distilled water. Insoluble material was removed by centrifugation.
Cells and viruses
Two virus strains were tested to evaluate the antiviral activity of B. bellinii and B. subtomentosus: Herpes Simplex Virus type 2 (HSV-2) and Coxsackie Virus B type 3 (CVB-3). These viruses were adapted to Vero (African green monkey kidney) cell line in DMEM supplemented with 2% FBS and containing 100 µg/mL streptomycin, 100 units/mL penicillin, and 0.25 µg/mL amphotericin B. The Vero cells were grown in the same medium with 5% FBS at 37 °C under humidified 5% CO2. Vero cell line (ATCC® CCL-81™) and the HSV-2 clinical isolate were obtained from the Pasteur Institute of Tunis, Tunisia, and the CVB-3 clinical isolate was kindly provided by the Faculty of Pharmacy of Monastir, Tunisia.
Virus titration
HSV-2 and CVB-3 strains were titrated by the endpoint dilution method and the plaque assay method as described previously by Reed and Muench (1938) and Hankins and Heam (1970), respectively, with some modifications. The titles were expressed as plaque forming unit/mL (PFU/mL) for HSV-2 and 50 tissue culture infectious dose 50%/mL (TCID50/mL) for CVB-3.
In vitro cytotoxicity assay
The cytotoxicity test was performed by the trypan blue dye exclusion method as described previously by Koyama and Miwa (1997) with some modifications. In brief, 100 µL of Vero cells (0.5 × 104) were seeded onto a 96-well plate (semi-confluent monolayer cells). The medium was replaced after 24 h of incubation at 37 °C (5% CO2) with 100 µL of different concentrations of B. bellinii and B. subtomentosus ECEs. After 3 days of incubation at 37 °C (5% CO2), monolayer cells were trypsinized and trypan blue solution (10% v/v) was added. Wells without ECEs were used as negative controls. The number of living cells was counted as compared to the cell control. The sample concentration that damages 50% of cell culture – which represents the 50% cytotoxic concentration (CC50) – was calculated by linear regression analysis from the dose-response curve.
In vitro antiviral activity
The anti-HSV-2 and anti-CVB-3 activity assays were performed by the plaque assay method and the MTT method as previously described by Sasaki et al. (2016) and Guo et al. (2006), respectively, with slight modifications.
For the anti-HSV-2 activity, 2 mL of Vero cells (105) were seeded onto a 35-mm dish (confluent monolayer cells). After 24 h of incubation at 37 °C (5% CO2), the medium was replaced by 2 mL of different concentrations of B. bellinii and B. subtomentosus ECEs from CC50/2 with 150 PFU of HSV-2 for 1 h. Then, the cells were washed with PBS to remove unabsorbed viruses and overlaid with the same extract concentration in DMEM counting 1% of methylcellulose. After 2 days of incubation at 37 °C (5% CO2), the medium was removed and the cells were stained with 0.3% crystal violet solution (0.3% w/v in 20% ethanol v/v), and then washed with PSB to remove the dye excess. The plaques were counted as compared to the virus control. The sample concentration that inhibits 50% of virus plaque – which represents the 50% inhibitory concentration (IC50) – was calculated by linear regression analysis from the dose-response curve. The selectivity index (SI) value was determined as the ratio of CC50/IC50.
For the anti-CVB-3 activity, 100 µL of Vero cells (104) were seeded onto a 96-well plate (confluent monolayer cells). After 24 h of incubation at 37 °C (5% CO2), the medium was replaced by 100 µL of different concentrations of B. bellinii and B. subtomentosus ECEs from CC50/2 with 10 TCID50 of CVB-3. Non-infected non-ECEs-treated cells and CVB-3-infected non-ECEs-treated cells were used as negative and positive controls, respectively. After 2 days of incubation at 37 °C (5% CO2), the medium was removed and the cells were covered with 100 µL of MTT solution (1 mg/mL) for 3 h at 37 °C. The MTT reagent is reduced by the mitochondrial succinate dehydrogenase of active living cells to formazan, of which the quantity is proportional to the number of living cells. Then, the MTT solution was removed and 100 µL of DMSO was added to each well for to dissolve the formazan crystals product from the cells. After 15 min, the microplate was read with an ELISA plate reader at 540 nm. The viral inhibition rate was determined following this formula: [(ODev – ODvc)/(ODcc-ODvc)] x 100, where ODev, ODvc and ODcc indicate the absorbance of the ECEs in presence of virus, the absorbance of virus control and the absorbance of cell control, respectively. The sample concentration that gave a viral inhibition rate of 50% corresponds to the IC50 and was calculated by linear regression analysis from the dose-response curve.
When SI ≥ 10 for HSV-2 (Dong et al. 2012) and ≥ 5 for CVB-3 (Abaza et al. 2019), the extract was considered as active.
Virus direct inactivation assay
HSV-2 (15.000 PFU) and CVB-3 (1.000 TCID50) virus suspensions were pre-treated with an equal volume of B. bellinii and B. subtomentosus ECEs at two different concentrations (IC50 and 10 x IC50). After 2 h of incubation, the mixture was diluted 100 fold to remove the effect of the extract on the virus replication and then added to confluent monolayer cells on 35-mm dishes / 96-well plates, respectively. After 2 days, the cells were subjected to plaque assay / MTT assay, as described in the antiviral activity assay. The percentages of viral infection were calculated as compared to the virus control.
Post-infection assay
Confluent monolayer cells on 35-mm dishes / 96-well plates were infected by 150 PFU of HSV-2 / 10 TCID50 of CVB-3, respectively. After 1 h of incubation, the cells were washed twice by PBS to remove unabsorbed viruses and then overlaid by B. bellinii and B. subtomentosus extracts with two concentrations (IC50 and 10 x IC50). After 6 h of incubation, the cells were washed twice with PBS to remove free compounds, and DMEM was added. After 2 days, the cells were subjected to a plaque assay / MTT assay, as described in the antiviral activity assay. The percentages of viral infection were calculated as compared to the virus control.
Viral adsorption and penetration assay
Confluent monolayer cells on 35-mm dishes / 96-well plates were incubated at 4 °C with 150 PFU of HSV-2 / 10 TCID50 of CVB-3, respectively. After 1 h, the cells were washed twice with PBS to remove unabsorbed viruses and then overlaid by B. bellinii and B. subtomentosus ECEs with two concentrations (IC50 and 10 x IC50) at 37 °C. After 2 h of incubation, the cells were washed twice with PBS to remove free compounds, and DMEM was added. After 2 days, the cells were subjected to a plaque assay / MTT assay, as described in the antiviral activity assay. The percentages of viral infection were calculated as compared to the virus control by deducting the rates obtained in this assay from those obtained in the post-infection assay.
Cell pretreatment assay
Confluent monolayer cells on 35-mm dishes / 96-well plates were pre-treated at 37 °C with B. bellinii and B. subtomentosus extracts at two concentrations (IC50 and 10 x IC50). After 2 h of incubation, the cells were washed twice with PBS to remove free compounds and then infected with 150 PFU of HSV-2 / 10 TCID50 of CVB-3, respectively. After 2 days, the cells were subjected to a plaque assay / MTT assay, as described in the antiviral activity assay. The percentages of viral infection were calculated as compared to the virus control by deducting the rates obtained in this assay from those obtained in the virus adsorption and penetration assay.
Isolation of the active compound
The isolation of the active compound was performed by TLC. An amount of 242 mg of B. bellinii and B. subtomentosus ECEs were deposed on a pre-coated silica gel 60 F254 glass plate (20 × 20 cm; Glass Backed TLC Extra Hard Layer 60 Å) and eluted with methanol/acetonitrile/H20 (3:3:0.5-v/v/v). After separation, the plate was air dried and observed under UV light at 254 nm. The visualized bands were scraped off and dissolved in the same mobile phase as used in TLC. After agitation for 15 min, the fractions were filtrated, dried, weighted, dissolved in 75% ethanol to a 10 mg/mL final concentration, and then evaluated for their cytotoxicity and antiviral activity.
Identification of the active compound
The analyses were performed using a Thermo Scientific LCQ FLEET system consisting of an LCQ FLEET ion trap mass spectrometer, a Surveyor MS Pump/Autosampler/PDA Detector (Thermo Fisher Scientific, Waltham, MA, USA) through an ESI source. The separation was obtained by using a Gemini® C18 110 A analytical column (150 × 2.00 mm i.d., 5 μm) and the pre-column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of aqueous formic acid at 0.1% and acetonitrile (solvent B) at 0.3 mL/min (the injection volume was 10 µL). A linear solvent gradient was used as follows: from 10% B to 95% B in 25 min with a final plateau of 3 min at 100% B. The ion trap operated in data-dependent, full scan (60–2000 m/z), and MSn mode to obtain fragment ions m/z with a collision energy of 35% and an isolation width of 3 m/z. The negative and positive parameters of the ion mode ESI source have been optimized to an ionization voltage of 5.0 kV, a capillary temperature of 320 °C, a capillary voltage of 32 V, a sheath gas flow rate of 25 arbitrary units (AU), and an auxiliary gas flow rate of 10 AU. Data was acquired using Thermo Excalibur 2.2 software (Thermo Fisher Scientific, MA, USA).
Statistical analysis
The experiments were carried out in triplicate and values are expressed as the mean ± standard deviation (SD).
Results
DNA sequencing analysis
A fragment of the DNA internal transcribed spacer sequences of mushroom samples was amplified and sequenced. The two Boletus strains could be identified up to species level from the available GenBank database. Molecular identification of the mushroom samples corresponds to morphological identification. The results were submitted to the GenBank database (Accession numbers: MN461459 and MN461458 for B. bellinii (synonym: Suillus bellinii) and B. subtomentosus (synonym: Xerocomus subtomentosus), respectively) with 100% similarity percentage.
Cytotoxic effect of the extracts on viability of Vero cells
Cell viability against different concentrations of B. bellinii and B. subtomentosus ECEs in Vero cells for 72 h of incubation was screened. In vitro cytotoxicity assays showed that the concentrations associated with 50% cytotoxicity concentration (CC50) of B. bellinii and B. subtomentosus ECEs on Vero cells were 2677 and 3414 µg/mL, respectively (Table 1). The CC50 values indicated that these extracts had no significant cytotoxic effect against Vero cells up to a concentration of 2 mg/mL.
The antiviral activity of extracts against HSV-2 and CVB-3
The Boletus ECEs were subjected to an in vitro plaque reduction assay and an MTT assay to evaluate the antiviral activity against HSV-2 and CVB-3, respectively. The 50% inhibitory concentration (IC50) of B. bellinii and B. subtomentosus ECEs exhibited a high inhibitory effect both against HSV-2 and CVB-3 (Table 1). B. bellinii ECE displayed relatively strong inhibitory activity as compared to B. subtomentosus ECE. The relevant values of the selectivity index (SI = CC50/IC50) of B. bellinii and B. subtomentosus ECEs obtained against HSV-2 were higher than those obtained against CVB-3 (SI > 600 for HSV-2 vs. < 100 for CVB-3).
Mode of antiviral action of B. bellinii and B. subtomentosus extracts
At a concentration of 10 x IC50, B. bellinii and B. subtomentosus ECEs completely inactivate HSV-2 by direct contact, inducing 100% of viral inhibition (Table 2). On the other hand, the ECE of both species revealed no virucidal effect against the non-enveloped virus CVB-3. Moreover, B. bellinii and B. subtomentosus ECEs demonstrated antiviral activity against both HSV-2 and CVB-3 during the early steps of virus replication, including virus-cell attachment, viral adsorption and penetration. However, after virus inoculation, the Boletus ECEs did not show any significant antiviral activity.
Isolation and identification of the active compound of B. bellinii extract
The thin layer chromatographic profiles of B. bellinii and B. subtomentosus ECEs were similar, characterized by the presence of two individualized bands (Fig. 1). Only the band with a retention factor (Rf) value of 0.88 exhibited antiviral activity against HSV-2 and CVB-3. This active band had the following characteristics: brown color under white light and blue color under UV light. The subjection of this fraction to a second TLC using the same protocol (with some variations in the proportion of the mobile phase) did not reveal other bands. Since the two extracts showed the same active band, only the one isolated from the most active extract (B. bellinii ECE) was scraped and subjected to HPLC-DAD-ESI-MS/MS analysis. The amount of the recovered active compound was 5.2 mg.
Figure 2 reports the UV chromatograms at 315 nm for B. bellinii active fraction. The HPLC profile showed a peak eluting at rotation time (Rt) 7.81, which gave a pseudomolecular ion at m/z 289 [M-H]− and 291 [M + H]+ in negative and positive ionization modes, respectively. An adduct with sodium was also revealed in positive ionization mode at m/z 313. The fragmentation in the negative ionization mode led to a base peak at m/z 135 due to the cinnamic acid residue. The MS/MS fragmentation pattern led to the putative identification of inonotusin A (Fig. 3) by comparison of its spectral data with those reported in the literature (Zan et al. 2011).
However, further analyses such as NMR spectroscopy will be conducted for the determination of the relative stereochemistry of the active compound.
Cytotoxicity, antiviral activity and mode of action of the active compound of B. bellinii extract
The purified active compound was evaluated for its cytotoxicity and antiviral activity. It showed a more cytotoxic effect than that of the native active extract (722 µg/mL versus 2677 µg/mL) and also a higher antiviral activity against both HSV-2 and CVB-3 (0.81 and 19.04 µg/mL versus 3.60 and 35.70 µg/mL, respectively) (Table 1). Therefore, the SI of the purified active compound increased compared to the native extract against HSV-2 (891 versus 744); however, the selectivity seems to decrease against CVB-3 since an augmented cytotoxicity was recorded.
The test of the mode of antiviral action performed on this compound showed similar results to those of the active extract. Better still, this compound showed a total inhibition of the virus (100%) during the virus penetration step against both HSV-2 and CVB-3 when it was tested at a concentration of 10 x IC50.
Discussion
The development of novel antiviral agents is required to prevent the continuous appearance of resistant viral strains and to control the propagation of new viral infections (Pekosz and Glass 2008). Earlier research suggested that mushrooms could be a potential natural source for the development of new antiviral drugs (Linnakoski et al. 2018). It has been reported that basidiomycete fruiting body and mycelia extracts are active against various viruses, including human immunodeficiency virus, enterovirus 71, herpes simplex virus, poliovirus, and influenza viruses (Table 3). Basidiomycetes produce a variety of secondary metabolites like polysaccharides and phenolic compounds with the potential to inhibit viral replication through diverse modes of action such as virucidal effect (Zhang et al. 2004), inhibition of integrase (Singh et al. 2003), inhibition of neuraminidase activity and binding site (Song et al. 2014), inhibition of virus adsorption and penetration (Faccin et al. 2007) and inhibition during viral replication as RNA synthesis and protein expression (Zhao et al. 2016). Boletus is a cosmopolitan mushroom genus of basidiomycetes with over 100 species. They are well known for their potential therapeutic and antimicrobial benefits (Garcia et al. 2022). Nevertheless, this is the first study to evaluate the antiviral activity of two edible Boletus species, B. bellinii and B. subtomentosus, against an enveloped DNA virus HSV-2 belonging to the Alphaherpesvirinae and a non-enveloped RNA virus CVB-3 belonging to the Picornaviridae.
The most crucial requirement for an antiviral agent is safety, and when searching for new drugs, it is important to look into potential side effects (Saxena et al. 2010). According to our research, B. bellinii and B. subtomentosus ECEs showed no significant cytotoxicity on the Vero cell line, with a CC50 value higher than 2000 µg/mL. The lower toxic effects imply that B. bellinii and B. subtomentosus ECEs are safe for use even at high doses (Ellan et al. 2019). On the other hand, our results provide evidence of the antiviral effect of Boletus extracts against HSV-2 and CVB-3, as indicated by their SI values (SI > 50 for CVB-3 and > 600 for HSV-2) (Dong et al. 2012; Abaza et al. 2019). In comparison to other natural extracts tested against HSV-2 and CVB-3 viruses, these findings suggest that Boletus extracts could be a very promising source for the development of new and less toxic antiviral compounds (Miaoxian et al. 2006; Hassan et al. 2015).
Phytochemical screening revealed that the ECEs of B. bellinii and B. subtomentosus contain two major fractions, of which only one showed significant anti-HSV2 and anti-CVB3 activities. Chromatographic separation and spectral analysis revealed that the active fraction contains a hispidin derivative named inonotusin A, which was first isolated from the methalonic extract of the fruiting bodies of Inonotus hispidus (Zan et al. 2011). Numerous researches have reported immunomodulatory (Gründemann et al. 2016), anti-allergic (Tamrakar et al. 2019), antioxidant (Zan et al. 2011), cytotoxic (Lee and Yun 2011), anti-inflammatory (Shao et al. 2015), anti-cancer (Lv et al. 2017), and anti-obesity (Tu and Tawata 2014) activities of mushroom hispidin. Additionally, it has been shown that hispidin demonstrated antiviral activities against diverse enveloped viruses, including influenza virus (IFV) types A and B (Awadh Ali et al. 2003; Hwang et al. 2015) and human immunodeficiency virus (HIV-1) (Singh et al. 2003). However, the antiviral activity of inonotusin A against HSV-2 and CVB-3 was demonstrated for the first time in this report. Furthermore, the presence of this molecule in Boletus was not described previously, even though hispidin and derivatives were mainly derived from the Hymenochaetaceae family, including Phellinus and the Inonotus genus (Ma et al. 2018). In addition, this compound has been rarely studied and the only report have described a potential antioxidant activity (Zan et al. 2011), as well as a moderate cytotoxic activity (IC50 = 19 µM) against a human breast carcinoma cell line (MCF-7) (Angelini et al. 2019).
In order to determine the mode of antiviral action of inonotusin A, the B. bellinii and B. subtomentosus ECEs were added at different stages during the viral infection cycle. The results indicated that both Boletus extracts exhibited anti-HSV-2 and anti-CVB-3 activities with two modes of action. Against CVB-3, these extracts acted at early steps of infection by inhibiting viral attachment and/or penetration through the cell membrane, while against HSV-2, these extracts acted directly on virus (virucidal effect), which could disturb the interaction of their envelope glycoproteins with target cell receptors, preventing them from binding to host cells.
To the best of our knowledge, this is the first study reporting the antiviral activity of B. bellinii and B. subtomentosus ECEs against CVB-3 and HSV-2. Indeed, this work reported for the first time the great antiviral potential of inonotusin A. These findings suggest a novel approach to using inonotusin A to control viral infections, with two distinct modes of action depending on the nature of the virus. In addition, our experiments demonstrated that this molecule directly inactivated HSV-2 particles and inhibited the CVB-3 viruses in the cell entry process. Further investigations are ongoing to verify the underlying antiviral mechanisms involved in these inhibitions, such as the nature of the virus attachment glycoproteins and the cellular receptors involved in this inhibition. Our results suggest that inonotusin A could be considered as a promising compound for the development of a novel and efficient naturally occurring antiviral agent with broad-spectrum.
Abbreviations
- Boletus bellini :
-
B. bellinii.
- Boletus subtomentosus :
-
B. subtomentosus.
- CC50 :
-
50% cytotoxic concentration.
- IC50 :
-
50% inhibitory concentration.
- SI:
-
Selectivity index.
- HIV:
-
Human immunodeficiency virus.
- FDA:
-
Food and Drug Administration.
- HSV-2:
-
Simplex Virus type 2.
- CVB-3:
-
Coxsackie Virus B type 3.
- DMSO:
-
Dimethyl sulfoxide.
- DMEM:
-
Dulbecco’s Modified Eagle’s medium.
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
- PBS:
-
Phosphate-buffered saline.
- FBS:
-
Fetal bovine serum.
- TLC:
-
Thin-layer chromatography.
- MEA:
-
Malt extract agar.
- MEB:
-
Malt extract Broth.
- ITS:
-
Internal transcribed spacer.
- NCBI:
-
National Center for Biotechnology Information.
- PCR:
-
Polymerase chain reaction.
- ECEs:
-
Extracellular crude extracts.
- TCID50 :
-
Tissue culture infectious dose 50%.
- Rf:
-
Retention factor.
- Rt:
-
rotation time.
- min:
-
Minute.
- h:
-
Hours.
- °C:
-
Degree celsius.
- µL:
-
Micro litre.
- µg:
-
Microgram.
- mL:
-
Milliliter.
- mm:
-
Millimeter.
- PFU:
-
Plaque-forming unit.
- w/v:
-
Weight by volume.
- v/v:
-
Volume by volume.
- OD:
-
Optic density.
- cm:
-
centimeter.
- nm:
-
Nanometer.
- UV:
-
Ultraviolet.
- AU:
-
Arbitrary units.
- SD:
-
Standard deviation.
References
Abaza L, Bouslama L, Benzekri R, Trabelsi N, Taamallia A, Jallouli S, Zarrouk M (2019) Isolation of an antiviral compound from Tunisian olive twig cultivars. Microb Pathog 128:245–249. https://doi.org/10.1016/j.micpath.2019.01.012
Amzat J, Razum O (2018) Towards a Sociology of Health Discourse in Africa: Traditional medicine in Africa. Springer International Publishing AG, Switzerland (CH)
Angelini P, Girometta C, Tirillini B, Moretti S, Covino S, Cipriani M, D’Ellena E, Angeles G, Federici E, Savino E (2019) A comparative study of the antimicrobial and antioxidant activities of Inonotus hispidus fruit and their mycelia extracts. Int J Food Prop 22:768–783. https://doi.org/10.1080/10942912.2019.1609497
Antonio AS, Wiedemann LSM, Veiga-Junior VF (2020) Natural products’ role against COVID-19. RSC Adv 10:23379–23393. https://doi.org/10.1039/D0RA03774E
Awadh Ali NA, Mothana RA, Lesnau A, Pilgrim H, Lindequist U (2003) Antiviral activity of Inonotus hispidus. Fitoterapia 74(5):483–485. https://doi.org/10.1016/S0367-326X(03)00119-9
Cardozo FTGS, Camelini CM, Mascarello A, Rossi JM, Nunes RJ, Regina C, Barardi M, Mendonça MM, Simões CMO (2011) Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir Res 92:108–114. https://doi.org/10.1016/j.antiviral.2011.07.009
Dong CX, Hayashi K, Mizukoshi Y, Lee JB, Hayashi T (2012) Structures and anti-HSV-2 activities of neutral polysaccharides from an edible plant, Basella rubra L. Int J Biol Macromol 50:245–249. https://doi.org/10.1016/j.ijbiomac.2011.10.022
Ellan K, Thayan R, Raman J, Hidari KIPJ, Ismail N, Sabaratnam V (2019) Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: an in-vitro study. BMC Complement Altern Med 19:260–272. https://doi.org/10.1186/s12906-019-2629-y
Faccin LC, Benati F, Rincão VP, Mantovani MS, Soares SA, Gonzaga ML, Nozawa C, Carvalho RE (2007) Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett Appl Microbiol 45(1):24–28. https://doi.org/10.1111/j.1472-765X.2007.02153.x
Garcia J, Rodrigues F, Castro F, Aires A, Marques G, Saavedra MJ (2022) Antimicrobial, Antibiofilm, and Antioxidant Properties of Boletus edulis and Neoboletus luridiformis Against Multidrug-Resistant ESKAPE Pathogens. Front Nutr 8:773346. https://doi.org/10.3389/fnut.2021.773346
Gründemann C, Arnhold M, Meier S, Bäcker C, Garcia-Käufer M, Grunewald F, Steinborn C, Klemd AM, Wille R, Huber R (2016) Effects of Inonotus hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med 82(15):1359–1367. https://doi.org/10.1055/s-0042-111693
Guo JP, Pang J, Wang XW, Shen ZQ, Jin M, Li JW (2006) In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J Gastroenterol 12:4078–4081. https://doi.org/10.3748/wjg.v12.i25.4078
Hankins WA, Hearn HJ (1970) Direct assessment of viral aerosols on cell cultures. Appl Microbiol 20:284–285. https://doi.org/10.1128/am.20.2.284-285.1970
Hassan S, Masarčíková R, Berchová K (2015) Bioactive natural products with anti-herpes simplex virus properties. J Pharm Pharmacol 67:1325–1336. https://doi.org/10.1111/jphp.12436
Hwang BS, Lee IK, Choi HJ, Yun BS (2015) Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg Med Chem Lett 25(16):3256–3260. https://doi.org/10.1016/j.bmcl.2015.05.081
Klofac W (2013) A world-wide key to the genus Suillus. Osterr Z Pilzkd 22:211–278
Koyama AH, Miwa Y (1997) Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J Virol 71(3):2567–2571. https://doi.org/10.1128/JVI.71.3.2567-2571.1997
Lee IK, Yun BS (2011) Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot 64:349–359. https://doi.org/10.1038/ja.2011.2
Lindequist U, Niedermeyer THJ, Julich WD (2005) The Pharmacological Potential of Mushrooms. eCAM 2(3):285–299. https://doi.org/10.1093/ecam/neh107
Linnakoski R, Reshamwala D, Veteli P, Escribano MC, Vanhanen H, Marjomäki V (2018) Antiviral Agents from Fungi: Diversity, Mechanisms and Potential Applications. Front Microb 9:1–18. https://doi.org/10.3389/fmicb.2018.02325
Liu J, Yang F, Ye LB, Yang XJ, Timani KA, Zheng Y, Wang YH (2004) Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Ethnopharmacol 95(2–3):265–272. https://doi.org/10.1016/j.jep.2004.07.010
Lv LX, Zhou ZX, Zhou Z, Zhang LJ, Yan R, Zhao Z, Yang LY, Bian XY, Jiang HY, Li YD et al (2017) Hispidin induces autophagic and necrotic death in SGC-7901 gastric cancer cells through lysosomal membrane permeabilization by inhibiting tubulin polymerization. Oncotarget 8(16):26992–27006. https://doi.org/10.18632/oncotarget.15935
Ma G, Yang W, Zhao L, Pei F, Fanga D, Hu Q (2018) A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci Hum Well 7:125–133. https://doi.org/10.1016/j.fshw.2018.05.002
Matsuhisa K, Yamane S, Okamoto T, Watari A, Kondoh M, Matsuura Y, Yagi K (2015) Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem Biophys Res Commun 462(1):52–57. https://doi.org/10.1016/j.bbrc.2015.04.104
Miaoxian S, Yaolan L, Kam TL, Yingzhou C, Ting L, Runzhi C, Vincent ECO (2006) Antiviral activity and constituent of Ardisia Chinensis Benth against Coxsackie B3 virus. Phytother Res 20:634–639. https://doi.org/10.1002/ptr.1912
Nguyen TL, Chen J, Hu Y, Wang D, Fan Y, Wang J, Abula S, Zhang J, Qin T, Chen X et al (2012) In vitro antiviral activity of sulfated Auricularia auricular polysaccharides. Carbohydr Polym 90(3):1254–1258. https://doi.org/10.1016/j.carbpol.2012.06.060
Ouali Z, Compagno R, Sbissi I, Gargano ML, Rhaiem A, Ben Naceur M, Venturella G, Jaouani A (2018) A preliminary check list of macromycetes in northern Tunisia. P Biosyst 152(1):31–58. https://doi.org/10.1080/11263504.2016.1244119
Patridge E, Gareiss P, Kinch MS, Hoyer D (2015) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21(2):204–207. https://doi.org/10.1016/j.drudis.2015.01.009
Pekosz A, Glass G (2008) Emerging Viral Diseases. Md Med 9:11–16
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Hyg 27:493–497. https://doi.org/10.1093/oxfordjournals.aje.a118408
Reis FS, Heleno S, Barros L, Sousa M, Martins A, Santos-Buelga C, Ferreira ICFR (2011) Toward the Antioxidant and Chemical Characterization of Mycorrhizal Mushrooms from Northeast Portugal. J Food Sci 76(6):824–830. https://doi.org/10.1111/j.1750-3841.2011.02251.x
Rincão VP, Yamamoto KA, Ricardo NM, Soares SA, Meirelles LD, Nozawa C, Linhares RE (2012) Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virol J 37:1–6. https://doi.org/10.1186/1743-422X-9-37
Roy BG (2017) Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir Chem Chemother 25(2):20–52. https://doi.org/10.1177/2040206617705500
Santoyo S, Ramírez-anguiano AC, Aldars-garcía L, Reglero G, Soler-rivas C (2012) Antiviral activities of Boletus edulis, Pleurotus ostreatus and Lentinus edodes extracts and polysaccharide fractions against Herpes simplex virus type 1. J Food Nutr Res 51:225–235
Sasaki K, Hayashi K, Matsuya Y, Sugimoto K, Lee JB, Kurosaki F, Hayashi T (2016) In vitro and in vivo antiherpetic effects of (1R,2R)-1-(5′-methylful-3′-yl)propane-1,2,3-triol. J Nat Med 70(2):217–224. https://doi.org/10.1007/s11418-016-0964-6
Saxena SK, Saxena S, Saxena R, Swamy MLA, Gupta A, Nair MPN (2010) Emerging Trends, challenges and prospects in antiviral therapeutics and drug development for infectious diseases. Elec J Biol 6:26–31
Shao HJ, Jeong JB, Kim KJ, Lee SH (2015) Anti-inflammatory activity of mushroom-derived hispidin through blocking of NF-κB activation. J Sci Food Agric 95(12):2482–2486. https://doi.org/10.1002/jsfa.6978
Singh SB, Jayasuriya H, Dewey R, Polishook JD, Dombrowski AW, Zink DL, Guan Z, Collado J, Platas G, Pelaez F et al (2003) Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites. J Ind Microbiol Biotechnol 30(12):721–731. https://doi.org/10.1007/s10295-003-0101-x
Song AR, Sun XL, Kong C, Zhao C, Qin D, Huang F, Yang S (2014) Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch Virol 159(4):753–760. https://doi.org/10.1007/s00705-013-1857-6
Tamrakar S, Fukami K, Parajuli GP, Shimizu K (2019) Antiallergic Activity of the Wild Mushrooms of Nepal and the Pure Compound Hispidin. J Med Food 22(2):225–227. https://doi.org/10.1089/jmf.2018.4267
Teplyakova T, Kosogova T (2015) Fungal Bioactive Compounds with Antiviral Effect. J Pharm Pharmacol 3:357–371. https://doi.org/10.17265/2328-2150/2015.08.001
Tu PT, Tawata S (2014) Anti-obesity effects of hispidin and Alpinia zerumbet bioactives in 3T3-L1 adipocytes. Molecules 19(10):16656–16671. https://doi.org/10.3390/molecules191016656
Zan LF, Qin JC, Zhang YM, Yao YH, Bao HY, Li X (2011) Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem Pharm Bull 59(6):770–772. https://doi.org/10.1248/cpb.59.770
Zhang L, Hu Y, Duan X, Tang T, Shen Y, Hu B, Liu A, Chen H, Li C, Liu Y (2018) Characterization and antioxidant activities of polysaccharides from thirteen Boletus mushrooms. Int J Biol Macromol 113:1–7. https://doi.org/10.1016/j.ijbiomac.2018.02.084
Zhang M, Cheung PC, Ooi VE, Zhang L (2004) Evaluation of sulfated fungal beta-glucans from the sclerotium of Pleurotus tuber-regium as a potential water-soluble anti-viral agent. Carbohydr Res 339(13):2297–2301. https://doi.org/10.1016/j.carres.2004.07.003
Zhang W, Tao J, Yang X, Yang Z, Zhang L, Liu H, Wu K, Wu J (2014) Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem Biophys Res Commun 449(3):307–312. https://doi.org/10.1016/j.bbrc.2014.05.019
Zhao C, Gao L, Wang C, Liu B, Jin Y, Xing Z (2016) Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr Polym 144:382–389. https://doi.org/10.1016/j.carbpol.2015.12.005
Money NP (2016) Fungal diversity. In: The Fungi 3rd edn. Elsevier, Academic Press, pp 1-36.
Acknowledgements
The authors thank Pr. Hela KALLEL and Mrs Ahlem BEN YAHIA (Pasteur Institute of Tunis, Tunisia) for providing Vero cell line and HSV-2 clinical isolate, respectively, and Dr. Hela JAIDANE (Faculty of Pharmacy of Monastir, Tunisia) for providing CVB-3 clinical isolate.
Author information
Authors and Affiliations
Contributions
Conceptualization and Investigation: [Soumaya Boudagga]; Formal Analysis: [Lamjed Bouslama] and [Adele Papetti]; Validation: [Raffaella Colombo]; Data curation: [Fatma Arous]; Supervision: [Atef Jaouani]; Writing: [Soumaya Boudagga] and [Lamjed Bouslama].
Corresponding author
Ethics declarations
Statements and declarations
The authors did not receive support from any organization for the submitted work.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boudagga, S., Bouslama, L., Papetti, A. et al. Antiviral activity of Inonotusin A an active compound isolated from Boletus bellinii and Boletus subtomentosus. Biologia 77, 3645–3655 (2022). https://doi.org/10.1007/s11756-022-01219-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01219-z