Abstract
International and national protection strategies and directives focus mainly on macroscopic organism and attempt to maintain their endangered habitats. However, microscopic communities are also threatened by decreasing biodiversity and many species including freshwater algae can disappear without even knowing they were present in the habitat. Defining rarity of microscopic taxa is not easy. The species’ rarity is based on detailed knowledge of distribution and abundance of species. But only limited information is available about rare algal species especially in a given ecoregion. Reducing the data gaps, here, we present altogether 20 phytoplankton taxa rare in Hungary: three species of Chlorophyceae, eight species of Trebouxiophyceae, two taxa of Euglenophyceae, one-one species of Cyanobacteria, Bacillariophyceae and Mediophyceae and three species of Xanthophyceae. One of them, the Cylindrotheca gracilis is on the Hungarian Red List. Physical and ecological characteristics of standing waters where these species were found as well as their former occurrence all over the world are also reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quest to understand how the world can work always presents new challenges for researchers. In the twentieth century, tasks had to be solved were under significant social pressure. From the middle part of the twentieth, eutrophication has been become the one of the most important tackled tasks both in local and global scales (Reynolds 2006). The surplus nutrient supply, mainly phosphorus and nitrogen, can cause undesirable changes in water quality (Istvánovics 2009). Eutrophication can modify the dynamics of phytoplankton with increasing algal biomass and blooming cyanobacteria resulting alteration in the species composition (Reynolds 2006). Recently, the drastic changes of climate, primarily hydrological drought or flash floods, cause deterioration of the quantity and quality of surface waters (Murdoch et al. 2000, Mosley 2015). In freshwaters, the structure of communities living here is significantly changed by the decreasing water level coupled with the increasing nutrient concentration, by the increasing water temperature, by the sediment resuspension and also by the stratification. Finally, these changes result biodiversity loss and habitat degradation (Mosley 2015).
Since eukaryotic algae and cyanobacteria play pivotal role in functioning of freshwater ecosystems, any changes in their structure can finally alter the composition of the whole ecosystem (Reynolds 2006). Thus, planktic algae and cyanobacteria are used to describe the status of habitats and to predict different disturbation (Juráň and Kaštovský 2019). These acts requires detailed knowledge about local and worldwide distribution of taxa, especially in case of rare ones (Juráň and Kaštovský 2019). Defining rarity of microscopic taxa including algae, however, is not easy. The species’ rarity is based on the distribution and the abundance of species, more precisely, the lack of actual distribution data (Molina 2013). Geographic range, abundance and habitat specialization were used to classify species into rarity categories and identify as rare or common (Molina 2013). But again, it is important to emphasize that the current distribution of taxa should be defined both at local and global scales to conclude whether taxa can be considered as rare or not.
Recently, the identification of planktic algae at species level can be carried out either by traditional, microscope method or by using modern, genetic tools, but both of them have still shortcomings (Bornet et al. 2004). The genetic-based methods are not effective for the whole planktic microflora yet, mainly because the databases are strongly incomplete (Bornet et al. 2004). On the other side, the microscopic-based methodology have to face difficulties such as (i) instrument side, light and/or inverted microscope with appropriate magnification is essentially needed; (ii) human side, a specialist with adequate knowledge who can determinate even more than 200 species from one lake during a season; (iii) state-of-art literature; (iv) time side, it takes many time to prepare one sample even in monitoring process; and (v) sample side, sufficient samples are also needed (Prof. F. Hindák pers. comm.). All of these criteria should be met in order to have reliable knowledge about actual distribution of a species.
In Hungary, there is a tradition of studying algology: At the end of 1970’s intensive studies were carried out with special attention to algae of rivers (Tamás 1949, Uherkovich 1959, Szemes 1969), lakes (Szabados 1938, Hortobágyi 1943, Vízkelety 1987), moors (Halász 1944), sodic lakes (Varga 1956, Kiss 1975), bogs (Palik 1940, Uherkovich 1962), caves (Kol 1964, Palik 1966) or springs (Palik 1957, Kol 1968). In the last decades, however, the scientific needs are changed and primarily focused on WFD-based researches. Thus, with some exception (e.g. Buczkó and Rajczy 1989, Padisák et al. 2003, Szabó et al. 2004), extensive taxonomical studies on algal flora of main rivers and standing waters have become sporadic in Hungary.
In this paper, a survey of data of Hungarian lakes and ponds is presented focusing on the ecological and taxonomical knowledge of rare algae.
Materials and methods
Study area
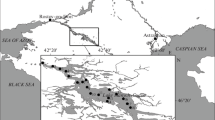
During the Hungarian surveillance monitoring program, almost 60 standing waters with catchment area greater than 50 ha were investigated in 2019 from April to September. In this article those standing waters are presented which contains rarely mentioned taxa according to the Hungarian national identification manuals (see later) (Fig. 1; Table 1 and Supplementary Table 1). The chosen lakes belong to small lakes, reservoirs, oxbow lakes and gravel pit lakes from very shallow to deep (30 water bodies; Table 1).
Location of standing waters where rare species were found. Numbers indicate the sampling sites (see in Supplementary Table 1)
Sampling methods
Phytoplankton and water samples were taken from euphotic layer of pelagical zone (calculated from 2 × Secchi depth) of the deepest part of lakes. Sampling was carried out by a tube sampler from a boat. Phytoplankton samples were preserved in the field with Lugol’s solution. Water samples were kept at 4 °C in a cooler bag during the transportation to the laboratory for further analyses. Conductivity (COND–µS cm− 1), pH, dissolved oxygen concentration (DO–mg L− 1), oxygen saturation (OS–%) and water temperature (T–°C) were measured by a portable-multiparameter digital meter (HQ30d, Germany) in situ. Analyses of other chemical parameters were carried out according the Hungarian national and international guidelines: nitrite (NO2− in mg L− 1, MSZ 1484-13:2009 (2009)), nitrate (NO3− in mg L− 1, MSZ 1484-13: 2009 (2009)), ammonium (NH4+ in mg L− 1, MSZ ISO 7150-1:1992 (1992)), total nitrogen (TN in mg L− 1, MSZ 12750-20:1972 (1972)), soluble reactive phosphorus (SRP in mg L− 1, MSZ EN ISO 6878:2004 (2004)), total phosphorous (TP in mg L− 1, MSZ 1484-3:2006 (2006); EPA 6020 A:2007 (2007)), chlorophyll a (Chl-a in µg L− 1, MSZ ISO 10260:1993 (1993)), biological oxygen demand (BOD in mg L− 1, MSZ EN 1899-1: 2000 (2000)), chemical oxygen demand (CODPs in mg L− 1, MSZ 448−20:1990 (1990)); hydrogencarbonate (HCO3− in mg L− 1; MSZ 448−11:1986), chloride (Cl− in mg L− 1, MSZ 448−15:1982 (1982)), soluble reactive silica (Si in µg L− 1, MSZ 1484-3:2006 (2006); EPA 6020 A:2007 (2007)) and total suspended solids (TSS in mg L− 1, MSZ 12750-6:1971 (1971)).
Trophic status of the studied standing waters was classified according to OECD (1982).
Phytoplankton
Phytoplankton samples were investigated with LEICA DMIL inverted microscope in 400-fold magnification. Species were documented by Canon EOS digital camera and by drawings. Most recent accepted name of algae was considered by AlgaeBase (Guiry and Guiry 2020). Curiosity of the microflora was based on previous occurrences of the taxa published in Hungarian identification manuals (Németh 1997, Schmidt and Fehér 1998, 1999, Grigorszky et al. 1999) and on the Hungarian Red List of Algae (Németh 2005).
The previous (from literature data) and the new occurrences (our observations) of taxa in Hungary were used to classify taxa into rarity scale categories The scale was similar to Juráň’s (2017) categories, who has described three categories for photosynthetic euglenoids of Check Republic. These categories are based on the number of local distributional data: (1) common (more than 15 localities), (2) rare (5 to 15 localities) and (3) very rare (maximally 4 localities).
Results
The physical and chemical parameters of the studied lakes and ponds are presented in Electronic Supplementary Material 1. While most of them can be categorized as hypertrophic or eu,-hypertrophic, Nyékládháza pit lake was the only standing water which is oligo,-mezotrophic. Additionally, Hasznosi reservoir and Kiskunlacháza-2 lake belong to eu,-mezotrophic.
In this paper 20 taxa, rare in Hungary are introduced: Chlorophyceae (3 species), Trebouxiophyceae (8 species), Euglenophyceae (2 species), Dinophyceae (1 species), Cyanobacteria (1 species), Bacillariophyceae (1 species), Mediophyceae (1 species), Xanthophyceae (3 species). Only one, the Cylindrotheca gracilis (Brébisson ex Kützing) Grunow is on the Hungarian Red List.
Bacillariophyceae.
* Cylindrotheca gracilis (Brébisson ex Kützing) Grunow (Fig. 2a).
Drawings of species rare in Hungary: a Cylindrotheca gracilis, b Desmatractum indutum, c Stauridium privum, d Tetrastrum triacanthum, e Nodularia spumigena, f Kolkwitziella acuta, g Trachelomonas bacillifera var. minima, h Trachelomonas woycickii var. pusilla, i Chaetoceros muelleri, j Coronastrum ellipsoideum (apical view). Scale bar 10 μm
Drawings of species rare in Hungary: a Closteriopsis longissima, b Dicloster acuatus, c Granulocystopsis coronata, d Granulocystopsis decorata, e Juranyiella javorkae, f Lagerheimia marssonii, g Paradoxia multiseta, h Bumilleriopsis verrucosa, i Centritractus brunneus, j Centritractus ellipsoideus. Scale bar 10 μm
Reference: Krammer and Lange-Bertalot (1988): p: 391, T 87: Fig. 3.
Description: Frustules very narrow, elongate with drawn-out ends and longitudinal spiraling of the cell.
Dimensions: 100 × 6 μm.
Similarity: The shape of the cell (spindle-like form, torsion of 2 and half twists and sub-capitate ends) is very unique, but the frustules can be easily collapsed.
Previous occurrences in Hungary: Fertő (Padisák 1984), Borsodi-dűlő (Stenger-Kovács and Lengyel 2015), Duna (Buczkó 1988).
New occurrence in Hungary: Kiskunlacháza-2 lake.
Ecological data: We found this species in a gravel pit lake with a few individuals. This species is listed into the Vulnerable (VU) category based on the Hungarian Red List of Algae (Németh 2005). Distribution is worldwide both in marine coasts and in freshwaters with high conductivity (Guiry and Guiry 2020).
Chlorophyceae.
Desmatractum indutum (Geitler) Pascher (Fig. 2b).
Reference: Komárek and Fott (1983): p. 261, Fig. 77:3.
Description: Cells are spindle-shaped pulled-out with fine long ends. Inner cell is ellipsoidal. Often a mucous membrane is presented but we didn’t recognize it. Superficial rib is longitudinal, colorless or brown color.
Dimensions: protoplasm: 3–5 × 4–6 μm.
Similarity: D. indutum is similar to Desmatractum delicatissimum, of which cell wall is smooth, cell halves goes gradually from the equatorial level to the pointed ones. While D. indutum has longitudinal superficial ribs and cell suddenly pulled out into a long process.
Previous occurrences in Hungary: Szeremlei Duna-branch, Buzsák fish pond, Ferenc-Tápcsatorna Hercegszántód, Tiszaalpári oxbow, Tiszaug oxbow, Tőserdő oxbow Schmidt and Fehér (1999); Babat fish pond (Hajdú 1976).
New occurrence in Hungary: Kenézi-morotva, Marótzugi-Holt-Körös, Tiszatarjáni-Holt-Tisza.
Ecological data: In this study, it has occurred sporadically mainly in oxbow lakes with a few individuals. It has worldwide distribution. For more details, see in Supplementary Table 2.
Stauridium privum (Printz) Hegewald (Fig. 2c).
Reference: Komárek and Fott (1983): p. 293, Fig. 86:2.
Description: Outer cell wall is straight or slightly concave, cell is 3–6 × 4–10 μm. Mostly 4-celled coenobia are appeared, but 8-celled coenobia can be also observed.
Dimensions: 8 × 8 μm.
Similarity: Under light microscope the 4 celled coenobium is similar to Crucigenia tetrapedia (Kirchner) Kuntze. However, the cell of S. privum is triangular, sometimes almost boomerang-like and the outer cell wall of the young cells is concave. In contrast, coenobium of C. tetrapedia is rectangle, cell walls are straight or just a bit concave. Under electron microscope the cell wall surface of S. privum is visibly sculptured, while in the case of C. tetrapedia it is smooth (Kowalska and Wołowski 2010).
Previous occurrences in Hungary: no earlier data from Hungary.
New occurrence in Hungary: Csórréti reservoir.
Ecological data: First occurrence in Hungary. Previously it was found in clear nordic lakes and peat bogs in the northern hemisphere. For more details, see in Supplementary Table 2.
Tetrastrum triacanthum Korshikov (Fig. 2d).
Reference: Komárek and Fott (1983): p. 771, Fig. 214:4.
Description: Coenobia is 4-celled, cells are square-shaped. One pyrenoid is visible. Central spine is long, while the 2 lateral spines are smaller and equally long.
Cell dimensions: 3–4 μm:
Similarity: It is different from the other Tetrastrum by the number and length of spines. Only the T. peterfii Hortobágyi species has 1–3 spines, but their spines are equal long and stand irregular.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Vadása-lake, Tisza: Szolnok-Szeged, Tiszaugi-oxbow, Tőserdő oxbow, based on T-Krasznai et al. (2013): Túr, Kishódos, Tiszadob, Felső Darab-Tisza oxbow.
New occurrence in Hungary: Boroszlókerti oxbow.
Ecological data: We found this species in a hypertrophic oxbow lake with a few individuals. This species is mentioned sporadically in the literature. For more details, see in Supplementary Table 2.
Cyanobacteria.
Nodularia spumigena Mertens ex Bornet & Flahault (Fig. 2e).
Reference: Komárek (2013): p. 909, Fig. 1177.
Description: In our samples, the filaments are straight, solitary and the aerotopes are missing. It has constricted at the cross-walls, not attenuated towards ends, sheath thick, colorless. Cells are discoid or barrel-shaped, heterocytes are oval, akinetes are almost spherical.
Dimensions: Filaments 200–230 × 8–8.4 μm, cells 8–8.4 × 3–4 μm.
Similarity: It is similar to Nodularia litorea Thuret ex Komárek, M.Hübel, H.Hübel & Smarda, which is wider (10–16 μm) then N. spumigena, and to Nodularia baltica Komárek, M.Hübel, H.Hübel & Smarda, which is shorter (4.5–7.2 μm) then N. spumigena. All of them can be straight or curved.
Previous occurrences in Hungary based on Feldöldi (1972): Duna, Nagyfai oxbow.
New occurrence in Hungary: Soponyai Sós-tó.
Ecological data: Here, the species was found in a soda lake with a few individuals. However, it is cosmopolitan and previously it was occurred in different waters with high content of minerals (Komárek 2013).
Dinophyceae.
Kolkwitziella acuta (Apstein) Elbrächter (Fig. 2f).
Reference: Moestrup and Calado (2018): p. 288, Fig. 249.
Description: The shape of cell is asymmetric. The epicone is conical or slightly concave, and extended into a short apical horn, while the hypocone is rounded. Chloroplast is absent. It is heterotrophic and cyst-forming species.
Dimensions: 35 × 35–40 μm.
Similarity: It has a unique plate formula (Po, X, 4’, 2a, 7’’, 3c + t, ?s, 5’’’, 1’’’’) and a characteristic shape of the cell.
Previous occurrences in Hungary according to Grigorszky et al. (1999): Balaton, small waters near Kőszeg, Nagyfai-Tisza oxbow.
New occurrence in Hungary: Faddi-Holt-Duna, Nyékládháza gravel pit lake, Délegyházi-lake, Leveleki reservoir.
Ecological data: It has occurred with a few individuals, mostly in summer plankton of oxbow lakes and reservoirs in our samples. Generally, it was rarely mentioned from larger eutrophic lakes. For more details, see in Supplementary Table 2.
Euglenophyceae.
Trachelomonas bacillifera Playfair var. minima Playfair (Fig. 2g).
Description: The whole surface of lorica is covered with short, blunt rod-like spines. The apical pore is without collar. Lorica is spherical or stubby ellipsoidal, 22–28 × 18–26 μm.
Dimensions: 28 × 25.2 μm.
Similarity: The Trachelomonas bacillifera var. minima is similar to Trachelomonas bacillifera f. sparsispina Deflandre, which has scattered and irregularly distributed spines. In addition, T. bacillifera var. minima is also similar to Trachelomonas bacillifera var. collifera Huber-Pestalozzi, which is differed from the other varieties by its apical pole with a short collar.
Previous occurrences in Hungary based on Németh (1997): Balaton, Barcsi Ősborókás, Buzsáki halastó, Cinkotai kubikgödör, Kaposvár: pocsolya, Kiskunhalasi ősláp, Csikor-tó (Polgár), Sajó, Bodrog holtláp (Sárospatak), Nagyfai Tisza Holtág, Szentmihályteleki Holt-Tisza, Szolnoki Holt-Tisza.
New occurrence in Hungary: Boroszlókerti oxbow.
Ecological and worldwide distribution data: We found this species in a hypertrophic oxbow lake with few individuals. Trachelomonas bacillifera var. minima is cosmopolitan and occurs in different kind of water bodies, like lakes, ponds, ditches and peat-bogs (Wołowski and Hindák 2004, 2005). For more details, see in Supplementary Table 2.
Trachelomonas woycickii Koczwara (Fig. 2h).
Reference: Németh (1997): p. 37, Fig. 7.B.
Description: Lorica is spherical, dark brown, size is 11–15 μm, and is covered by short, fine spines. Apical pore is without collar; flagellum is 3 times longer than lorica length.
Dimensions: 11–11.2 μm.
Similarity: The T. woycickii is similar to Trachelomonas globularis (Averintsev) Lemmermann, which is covered with short thick spines.
Previous occurrences in Hungary: Balaton, Barcsi ősborókás, Belső-tó (Tihany), Cinkotai kubikgödör, Kiskunhalasi ősláp, Jakabhegyi-reservoir, Fehér-tó, Szentmihálytelek oxbow lake, Tiszafüred oxbow lake (Németh 1997) and Malom-Tisza oxbow lake (Tiszadob; Krasznai et al. 2010).
New occurrence in Hungary: Nagy-Morotva.
Ecological data: It was found in a hypertrophic oxbow lake. In the international literature, it was mentioned as a cosmopolitan species (Wołowski and Hindák 2005). For more details, see in Supplementary Table 2.
Mediophyceae.
Chaetoceros muelleri Lemmermann (Fig. 2i).
Reference: Krammer and Lange-Bertalot (1991): p. 391, T 80: Figures 1 and 2.
Description: It is a colonial species, but in our samples only single cell form was found. From each pole of valve 1–1 twisted setae emerged. Valves are cylindrical with elliptic base and are lightly silicified.
Dimensions: 12 × 5 μm.
Similarity: The shape of this algae is very unique.
Previous occurrences in Hungary: Duna (Schmidt 1994), Lake Velence (Ács et al. 1994), Lake Fertő tó (Padisák 1984), Szelider lake (Szemes 1959), Belső-tó, Tihany (Ponyi and Tamás 1964), Kunfehértó (Uherkovich 1970), Szelidi Lake (Schmidt 1975).
Occurrence in Hungary: Szelidi Lake (last mention from this lake was 45 years ago).
Ecological data: Here, we found this species in a high conductivity lake. It is cosmopolitan (Guiry and Guiry 2020). For more details, see in Supplementary Table 2.
Trebouxiophyceae.
Coronastrum ellipsoideum Fott (Fig. 2j).
Reference: Komárek and Fott (1983): p.749, Fig. 209: 4.
Description: Cells are characterized by ellipsoid shape with horn-like mother cell wall fragments. This fragment is connected to one pole of cell, more or less visible.
Cell dimensions: 10 × 4 μm.
Similarity: The colony of C. ellipoideum is reminded to Komarekia appendiculata (Chodat) Fott, because 4-celled coenobium with mother cell wall fragments is characteristic of both species. But the cells of K. appendiculata are egg-shape and the cells are connected inside.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Balaton.
New occurrence in Hungary: L-I lake, Békéscsabai Téglagyári gravel pit lake.
Ecological data: We found this species in hypertrophic and eu- hypertrophic lakes. Previously, it was found in different lakes, pond and rivers mostly in Europe. For more details, see in Supplementary Table 2.
Closteriopsis longissima (Lemmermann) Lemmermann (Fig. 3a).
Reference: Komárek and Fott (1983): p. 327, Fig. 175:3.
Description: Cell is solitary, slender and very long, needle-shaped (190–240(570) µm) with 2–16 pyrenoids.
Dimensions: 250–360 × 4 μm.
Similarity: Morphologically the Schroederia setigera is similar, but it has only 1 pyrenoid.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Balaton, Dráva, Duna, Szigetköz, Gödöllő, Vadása-tó (Hegyhátszentjakab), Kis-Balaton, Körösök, small waters near Kőszeg, Kovácsszénájai-lake (Mecsek-hg), Herman Ottó-lake, Jakabhegy reservoir, Öcsi Nagy-lake, Rába, Tisza, Kiskörei reservoir, Nagyfa-oxbow, Velencei lake, Zagyva.
New occurrence in Hungary: Tiszalúci oxbow.
Ecological data: In our study, the species occurred in hypertrophic oxbow lake with a few individuals. In the world, this species is cosmopolitan. For more details, see in Supplementary Table 2.
Dicloster acuatus C.-C. Jao, Y.S. Wei & H.C. Hu (Fig. 3b).
Reference: Komárek and Fott (1983): p. 811, Fig. 224:4.
Description: Elongated cells with long pointed apices are in a two-celled coenobium. When 2 or more pairs of cells stay together, they are wedged into each other.
Dimensions: 32 × 4 μm.
Similarity: The shape of the colony is very unique.
Previous occurrences in Hungary Schmidt and Fehér (1999): Szeremlei Duna-branch, Duna, Kis-Balaton, Rába, Szigetköz, Zala.
New occurrence in Hungary: Belső-Béda.
Ecological data: Here, it has occurred in a hypertrophic oxbow lake. It was mentioned, however, from the whole part of the world. For more details, see in Supplementary Table 2.
Granulocystopsis coronata (Lemmermann) Hindák var. coronata (Fig. 3c).
Reference: Komárek and Fott (1983): p. 497, Fig. 146:2.
Description: The cells were ellipsoid to oval, solitary and have chloroplast with pyrenoid. The formation of brown granules is occurred only in poles but granules are never forming crowns.
Dimensions: 6–8 × 4–4,5 μm.
Similarity: It is similar to Granulocystopsis elegans (Fott) Hindák, but in case G. elegans granules are visible both in the polar and in the equatorial part of the cell.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Sugovica, Budapest waterworks, Buzsák fish pond, Duna, Szelidi-tó, Lake Fertő, Cserta-Duna, Szigetköz, Tisza.
New occurrence in Hungary: Faddi-Holt-Duna, Tiszalúc oxbow, Tolnai-Déli-Holt-Duna, Tolnai-Északi-Holt-Duna.
Ecological data: It has sporadically occurred in hypertrophic oxbow lakes in our samples. It was found in different habitats, e.g. plankic and benthic of swamps, river, lakes in worldwide. For more details, see in Supplementary Table 2.
Granulocystopsis decorata (Svirenko) Tsarenko (Fig. 3d).
Reference: Komárek and Fott (1983): p. 497, Fig. 146:4.
Description: The cells are ellipsoid to oval, solitary. Parietal chloroplast with pyrenoid is visible. Only polar crowns of brown granules are observed.
Dimensions: 14 × 8.4 μm.
Similarity: It is similar to Granulocystopsis coronata var. coronata of which granules are never forming crowns.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Budapest waterworks, Duna, Maros.
New occurrence in Hungary: Nagybaracskai-Holt-Duna.
Ecological data: In this study, it was sporadically found in a hypertrophic oxbow lake. It is a rarely mentioned species in the international literature. For more details, see in Supplementary Table 2.
Juranyiella javorkae (Hortobágyi) Hortobágyi (Fig. 3e).
Reference: Komárek and Fott (1983): p. 543, Fig. 158: 9.
Description: The cell is lunate-shaped with widely rounded cell ends. The surface of cell wall is covered by brownish warts.
Dimensions: 11–20 × 3–5 μm.
Similarity: The cell shape of the J. javorkae is similar to Nephrochlamys and Kirchneriella species, but they have smooth cell wall.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Budapest waterworks, Buzsák fishpond, Duna, Hortobágy fishpond, Fehér-tó, Szigetköz, Tisza, Kiskörei reservoir.
New occurrence in Hungary: Szarvas-Békésszentandrási-holtágrendszer, L-I lake, Hórvölgyi reservoir, Peresi oxbow, Belső-Béda, Harangzugi-Holt-Körös.
Ecological data: It has sporadically occurred in eutrophic or hypertrophic lakes or oxbow lakes in our samples. Rarely mentioned species in the international literature. For more details, see in Supplementary Table 2.
Lagerheimia marssonii Lemmermann (Fig. 3f).
Reference: Komárek and Fott (1983): p. 471, Fig. 140: 5.
Description: Cell is singular and widely oval; the surface of the cell is smooth. It has altogether 6 spines: 4 equatorially placed spines and on the poles is 1–1 spine. The length of the spine is 9–10 μm (Hindák 1980), but in our materials the length of the spines was 5–8 μm (this “short spine length” form was earlier called Lagerheimia minor Fott).
Dimensions: 6–8 × 4–5 μm.
Similarity: It is similar to Lagerheimia wratislawiensis Schröder, but it has only 2 equatorial spines beside 1–1 polar spines.
Previous occurrences in Hungary based on Schmidt and Fehér (1999): Duna, Gödöllő fish pond, Karapancsai Főcsatorna, Szigetköz, Szolnok oxbow; furthermore, Babat fish pond (Hajdú 1976).
New occurrence in Hungary: Tiszalúc oxbow, Nagy-Morotva, Bátai- oxbow, Belső-Béda, Halásztelek-Túrtő-Harcsás-Holt-Körös.
Ecological data: It has occurred in hypertrophic oxbow lakes with a few individuals in our samples. It was rarely mentioned in the international literature and found mainly in reservoirs. For more details, see in Supplementary Table 2.
Paradoxia multiseta Svirenko (Fig. 3g).
Reference: Komárek and Fott (1983): p 231. Figure 67: 2.
Description: Drop or mace-shaped cells have on their surface bristles. The cells are in pair connecting with two leaf-like appendages. Chloroplast has 1 or 2 pyrenoids.
Dimensions: 12–16 × 4–5 μm.
Similarity: Paradoxia is similar to Ankyra. But there are several differences e.g. Paradoxia is characterized by coenobium, blunt cell pole, and presence of bristles, while Ankyra is always described by unicellular, acute cell pole and absent of bristles.
Previous occurrences in Hungary: Budapest waterworks, Duna, Sajó (Schmidt and Fehér 1999).
New occurrence in Hungary: Hasznosi reservoir, Tiszalúc oxbow.
Ecological data: We found only a few individuals of this species in a meso-eutrophic reservoir and a hypertrophic oxbow lake. However, it was found worldwide. For more details, see in Supplementary Table 2.
Xanthophyceae.
Bumilleriopsis verrucosa (Hortobágyi ex Loeblich, III) Ettl (Fig. 3h).
Reference: Ettl (1978): p. 391, Fig. 483.
Description: Cells are solitary, cylindrical, curved, divided into two parts, cell wall covered by brownish warts.
Dimensions: 3 × 12–16 μm.
Similarity: The cell wall of B. verrucosa is relatively rigid in contrast with Juranyiella javorkae, which has also brown warts on its surface.
Previous occurrences in Hungary: Buzsáki fishpond (Schmidt and Fehér 2001).
New occurrence in Hungary: Szarvas-Békésszentandrási-holtágrendszer, Halásztelek-Túrtő-Harcsás-Holt-Körös.
Ecological data: It has occurred in hypertrophic oxbow lakes. It was very rarely mentioned in the international literature. For more details, see in Supplementary Table 2.
Centritractus brunneus Fott (Fig. 3i).
Reference: Ettl (1978): p. 398, Fig. 492.
Description: Cell wall is thick and is covered by brownish warts. Cells are bent (more or less bean-like shaped) or straight, asymmetrical. The apical spine is longer than cell length.
Cell dimensions: 3–5 × 10–14 μm, spines 9–12 μm.
Similarity: It is similar to Centritractus selliferus Krienitz which has bean-like shaped, brown warts and the end of the cell wall has thickness.
Previous occurrences in Hungary Schmidt and Fehér (2001): Buzsák fish pond, Gödöllő fish pond, Vadása-tó.
New occurrence in Hungary: Félhalmi oxbow, Halásztelek-Túrtő-Harcsás-Holt-Körös.
Ecological data: It has occurred in eutrophic lakes with a few individuals in our study. It is a rarely mentioned species both in Hungary and in the international literature. For more details, see in Supplementary Table 2.
Centritractus ellipsoideus Starmach (Fig. 3j).
Reference: Ettl (1978): p. 401, Fig. 497.
Description: Cell is ellipsoid almost spherical; curved apical spine is longer than the cell length. Cell wall is smooth.
Dimensions of cell: 4–8 × 5–12 μm.
Similarity: It is similar to C. dubius Printz, which strait apical spine is shorter than the cell length. C. ellipsoideus is also similar to C. belonophorus Fott, but the cell wall of C. ellipsoideus was thicker than in C. belonophorus.
Previous occurrences in Hungary based on Schmidt and Fehér 2001: Buzsák fish pond.
New occurrence in Hungary: Boroszlókerti Holt Tisza (Gulács), Halásztelek-Túrtő-Harcsás-Holt-Körös, Kenézi-morotva, Marótzugi Holt Tisza (Gávavencsellő), Mocskos Duna (Homorúd), Nagy-Morotva, Szarvas-Békésszentandrási-holtágrendszer, Tiszaluc oxbow, Tiszatarján oxbow, Tunyogmatolcs reservoir.
Ecological data: It has occurred in eutrophic, mainly oxbow lakes, we found only a few individuals in the samples. It was sporadically mentioned in the international literature. For more details, see in Supplementary Table 2.
Discussion
Cultural eutrophication, when nutrient input is caused by human activities, is a widespread problem and it affects many freshwaters. According to the International Lake Environmental Committee 40–50% of lakes and reservoirs were eutrophicated in global scale (Istvánovics 2009). In Hungary 36.5% of our lakes, that are monitored as EU WFD required, are eutrophicated (2nd RBMPs). In oligo- and mesotrophic water bodies rare algae could be more expected than in more loaded ones reported by Juráň (2017). However, the most species presented in this study were found in hypertrophic and eu- or hypertrophic waters.
Only three species, Cylindrotheca gracilis, Paradoxia multiseta and Kolkwitziella acuta occurred in less loaded reservoir and gravel pit lakes (oligotrophic or mezotrophic). Among them the Cylindrotheca gracilis is the only species which was classified into vulnerable category based on Hungarian Red List (Németh 2005). C. gracilis is rarely mentioned in Hungary, however this species is cosmopolitan. This species was former called “brackish water species” (Christensen and Reimer 1968). The conductivity of the gravel pit lake where we found this taxon was high. In Hungary, Paradoxia multiseta had sporadic in the 20th century (Schmidt and Fehér 1998, Borics et al. 2016), despite of having widespread distribution. This species occurred only in low cell number in a reservoir and an oxbow lake. The Kolkwitziella acuta was formerly known as a permanent member of phytoplankton in Balaton and found other standing waters all the country (Grigorszky et al. 1999). Nowadays, K. acuta, which has special thecal plate scheme and its cyst indicates low salinities, is a very rare species both in Hungary and in the world (Mertens et al. 2015).
As we mentioned above, most of the rare species were found mostly in eu-, or hypertrophic standing waters. This supports the fact that rare species may also be present in eu-, or hypertrophic lakes. The distribution classification (common, rare and very rare categories) on the local scale were also used for classify the worldwide distribution data in a taxa rarity scale.
Some of the mentioned species have worldwide distribution. Furthermore, five of them, namely Chaetoceros muelleri, Closteriopsis longissima, Desmatractum indutum, Dicloster acuatus, Granulocystopsis coronata var. coronata, can be considered as common species in global scale. In spite of having common distribution in Hungary, Closteriopsis longissima has not mentioned since 30 years. It was occurred mostly in oxbow lakes, lakes and rivers. Now, it was detected in pelagial zone of eu-, hypertrophic oxbow, while Buczkó and Ács (1992) found this species on periphyton of green reed in the Szigetköz section of the Danube River. In form and size, Desmatractum indutum can be highly variable (Kiss 1978). It was usually found in oxbow lakes in Hungary (Schmidt and Fehér 1998). Here, this species also occurred in eu-, and hypertrophic oxbow lakes along the Tisza River and the Körös River. Granulocystopsis coronata var. coronata was formerly detected in planktic flora of oxbow lakes and large rivers as the Duna River and the Tisza River mainly in mid-summer period (Schmidt and Fehér 1998). Here, we found this taxon in oxbow lakes with connection of the mentioned rivers. Similar to our observations, Schmidt (1994) also reported sporadic occurrence of Chaetoceros muelleri in Hungary. This species is known as a marine/brackish algae also occurring in inland waters with high conductivity (Krammer and Lange-Bertalot 1991). Now, we found it in a shallow lake with high chloride content and also conductivity. In Europe, Dicloster acuatus was firstly reported from a Hungarian reconstructed wetland (Schmidt et al. 1991) and after that it was sporadically appeared in the Danube and its side-branches (Schmidt et al. 2003). Now, it was found in an oxbow lake of the Danube. In general, D. acuatus is sporadically found worldwide. According to Wołowski and Hindák (2004, 2005) Trachelomonas bacillifera var. minima and Trachelomonas woycickii are occurred widespread. Trachelomonas bacillifera var. minima was formerly found in standing waters with different characters all over our country (Németh 1997). Now, we found it in shallow, hypertrophic oxbow lakes. Trachelomonas woycickii is rarely mentioned in Hungary. Previously, T. woycickii was recorded mostly in different kind of standing waters. The new locality of this species, Nagy-Morotva, is a hypertrophic, shallow oxbow lake which is used for fishing.
Although Stauridium privum (syn. Pediastrum privum (Printz) Hegewald) has circumpolar boreo–alpine distribution and it is a common species (number of localities > 15), it was just sporadically mentioned from each country (Geriš 2004). From Hungary, S. privum has not been reported yet. Now, it was found in a small, shallow and eutrophic reservoir in the Mátra Mountains.
Some of the mentioned species are also rare both in Hungary and worldwide. Three of them Bumilleriopsis verrucosa, Centritractus ellipsoideus and Coronastrum ellipsoideum had only one earlier data from Hungary, from fish pond near Buzsák and from Lake Balaton respectively (Transdanubian part of the country). Here, these taxa appeared in shallow, mainly hypertrophic standing waters in the Transtisza region. Interestingly, Centritractus ellipsoideus was found in India under the ice cover of the Alpine Lakes of Arunachal Pradesh (Das 2016). Further worldwide rare taxa, Granulocystopsis decorate and Centritractus brunneus were published a few times according to the Hungarian identification manuals. The G. decorata was earlier reported from Hungarian large rivers such as the Danube River and the Maros River, now we found this species in an oxbow lake of the Danube River.
Three other species are also rare in the world, these species are Tetrastrum triacanthum, Juranyiella javorkae and Lagerheimia marssonii. Tetrastrum triacanthum was formerly described from oxbow lakes and slow flowing watercourses in Hungary (T-Krasznai et al. 2013). Here, we found only few specimens of this taxon in an oxbow lake. Lagerheimia marssonii was previously described from a fishpond near Gödöllő in North Hungary (Hajdú 1976), and rarely found in different waterbodies. Juranyiella javorkae was previously mentioned as rare species in Lake Kacsa that is a dead arm of the Tisza River (Kiss and Ács 2002). Now, we found it in oxbow lakes and reservoirs characterized by high trophic status. Based on our observations, the distribution of the latter two species, i.e. Centritractus ellipsoideus and Juranyiella javorkae, shows an expanding trend in the last decades.
Beside eutrophication the drastic changes of climate can endangered our waters and their species in global and local scale. The lack of precipitation, outflow or flushing and the strong evaporation can cause drastic changes in the water level. The water level reduction can lead the deterioration of water quality with increasing total nitrogen and phosphorus content and/or salinity of waters (Mosley 2015). Species, indicating this circumstances, usually are found in small waters (soda pans or small watercourses; Schmidt and Fehér 2001, Kókai et al. 2015, Stenger-Kovács and Lengyel 2015, Stenger-Kovács et al. 2016). Since climate models predict increase in frequency and intensity of extreme climatic events in the continental region (IPCC 2014, Babka et al. 2018), these small ponds and streams are endangered by droughts and flash floods (Stenger-Kovács et al. 2016, B-Béres et al. 2019). In this study, the Soponyai-Sós-tó desiccated regularly. Here, one species rare in Hungary, Nodularia spumigena was found. This is a planktic species occurring mainly in marine waters, but it is also known from inland waters with high conductivity (Komárek 2013). The pond where we found this species had the highest conductivity among the studied standing waters.
Nowadays waters are under-sampled in Hungary. Unfortunately, even if there are results of more waters, they haven’t been represented in public database. Our results indicated that less-mentioned species can be found with low sampling effort even during national monitoring program. To answer the question what makes rare a species, we have to clarify whether the locality is unique, our knowledge is poor about its distribution or we are not able to determine the given species with traditional methods. To solve this task, the maintenance of long term water quality monitoring programs is necessary (Mosley 2015).
Despite spreading knowledge of new species or new distribution data of rare species, the biodiversity of our waters are still less known. Hundreds of different species can live together in an average lake (Cotthingham 1996). Heraclitus said many years before “You cannot step into the same river twice”. This universal phrases can be also true for lakes. Although the average succession pattern of phytoplankton is known, it can differ from year to year (Scheffer 2004). The few dominant species or group can be more or less predictable, while the accompanying species can be rather erratic (Padisák 1993). To find the appropriate frequent and number of sampling we can define the diversity and structure of phytoplankton, it gives beauty for our works. The mentioned rare species can be easy determined, if we have up-to-date literatures, light microscope and an algologist. On the whole the systematic seasonal studies of different habitats are still necessary.
References
Ács É, Buczkó K, Lakatos G (1994) Changes in the mosaic-like water surfaces of the Lake Velence as reflected by reed periphyton studies. Studia Bot Hung 25:5–19
Babka B, Futó I, Szabó S (2018) Seasonal evaporation cycle in oxbow lakes formed along the Tisza River in Hungary for flood control. Hydrol Process 32:2009–2019. https://doi.org/10.1002/hyp.13126
B-Béres V, Tóthmérész B, Bácsi I, Borics G, Abonyi A, Tapolczai K, Török P (2019) Autumn drought drives functional diversity of benthic diatom assemblages of continental intermittent streams. Adv Water Resour 126:129–136. https://doi.org/10.1016/j.advwatres.2019.02.010
Borics G, Tóthmérész B, Várbíró G, Grigorszky I, Czébely A, Görgényi I (2016) Functional phytoplankton distribution in hypertrophic systems across water body size. Hydrobiologia 764:81–90. https://doi.org/10.1007/s10750-015-2268-3
Bornet B, Antoine E, Bardouil M, Marcaillou-Le Baut C (2004) ISSR as new markers for genetic characterization and evaluation of relationships among phytoplankton. J Appl Phycol 16(4):285–290. https://doi.org/10.1023/B:JAPH.0000047780.24787.93
Buczkó K (1988) The diatom collection of László Vida. Studia Bot Hung 20:77–94
Buczkó K, Ács É (1992) Preliminary studies on the periphytic algae in the branch–system of the Danube at Cikolasziget (Hungary). Studia Bot Hung 23:49–62
Buczkó K, Rajczy M (1989) Contributions to the flora of the Hungarian caves. II. Flora of three caves near Beremend, Hungary. Studia Bot Hung 21:13–25
Christensen CL, Reimer CW (1968) Notes on the Diatom Cylindrotheca gracilis (Breh. ex Kutz) Grun: Its Ecology and Distribution. Proc Iowa Acad Sci 75: 36–41
Cottingham KL (1996) Phytoplankton responses to whole – lake manipulations of nutrients and food webs. University of Wisconsin, Madison, 422 pp
Das SK (2016) Floristic study of algae under the ice covers in the alpine lakes of Arunachal Pradesh, India (Eastern Himalayas). Crypto Biodiv Assess 1/1:75–83. https://doi.org/10.21756/cba.v1i1.11021
EPA Method 6020A:2007 (2007) Inductively coupled plasma–Mass spectrometry, 30 pp
Ettl H (1978) Xanthophyceae. In: Ettl H, Gerloff J, Heynig H (eds) Süsswasserflora von Mitteleurope, Band 3/1. VEB Gustav Fischer Verlag, Jena, 530 pp
Feldöldi L (1972) Kékalgák (Cyanophyta) kishatározója. Vízügyi Hidrobiológia. VIZDOK. Bp. I. pp 279
Geriš R (2004) Pediastrum privum (PRINTZ) HEGEWALD in the Czech Republic. Fottea 4:63–66
Grigorszky I, Vasas F, Borics G (1999) A guide for the identification of Dinophyta taxa occurring in Hungary. KGI, Budapest. In Hungarian, 220 pp
Guiry MD, Guiry GM (2020) AlgaeBase. World–wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 31 May 2020
Hajdú L (1976) Data to the algal flora of Hungary II. Stud Bot Hung 11:17–34
Halász M (1944) Algologische Notizen zur Kenntnis der Moor–Formation des Komitates Somogy. Das Phytoplankton des Baláta–Sees. Annls hist.–nat. Mus natn hung 36:1–24
Hindák F (1980) Studies on the chlorococcal algae (Chlorophyceae). II. Biologicke Práce 26, VEDA, pp 195
Hortobágyi T (1943) Beiträge zur Kenntnis der im Boglárer Seston, Psammon und Lasion lebenden Algen des Balaton–Sees. Ann Inst Biol (Tihany) 15:75–127 (In Hungarian)
IPCC Intergovernmental Panel on Climate Change (2014) Climate Change: Mitigation of Climate Change. Summary for policy makers and technical summary. Available at https://www.ipcc.ch/pdf/assessment-report/ar5/wg3/WGIIIAR5_SPM_TS_Volume.pdf . Accessed 10.05.2018
Istvánovics V (2009) Eutrophication of lakes and reservoirs. In: Likens GE (ed) Encyclopedia of inland waters, vol 1. Elsevier, Oxford, pp 157–165
Juráň J (2017) The checklist of photosynthetic euglenoids (order Euglenales) of the Czech Republic: ecology, taxonomy, distribution. Phytotaxa 317(1):1–16. https://doi.org/10.11646/phytotaxa.317.1.1
Juráň J, Kaštovský J (2019) The procedure of compiling the Red List of microscopic algae of the Czech Republic. Biodivers Conserv 28:24992529. https://doi.org/10.1007/s10531-019-01792-x
Kiss I (1975) A Fülöpháza-környéki szikes tavak, a Szappanos-szék, a Zsíros-szék, a Hattyús-szék és a Kondor-tó mikroflórájának és mikrovegetációjának összehasonlító vizsgálata. Acta Acad. Paed. Szegediensis 1975: 3–35. In Hungarian
Kiss I (1978) Algological investigations in the dead–Tisza at Lakitelek–Tőserdő. Tiscia 13:27–47. In Hungarian
Kiss KT, Ács É (2002) Nature conservation oriented algal biodiversity monitoring investigations in the main arm and some dead arms of the River Tisza II. Phytoplankton International Association for Danube Research 34:163–171
Kókai Zs, Bácsi I, Török P, Buczkó K, T-Krasznai E, Balogh Cs TB, B-Béres V (2015) Halophilic diatom taxa are sensitive indicators of even short term changes in lowland lotic systems. Acta Bot Croatica 74:287–302. https://doi.org/10.1515/botcro-2015-0025
Kol E (1964) The microvegetation of a small ice–cave in Hungary. Int J Speleol 1:19–24
Kol E (1968) Algologische und hydrobiologische Quellen–unter–suchungen im Nördlichen Bakony–Gebirge. – Veszprém Megyei Múzeumok Közl 7:131–145. In Hungarian
Komárek J (2013) Cyanoprokaryota: III. In: Büdel B, Gärtner G, Krienitz L, Schagerl M (eds) Süßwasserflora von Mitteleuropa, Band 19/1. Springer Spektrum, Berlin – Heidelberg, p 1130
Komárek J, Fott B (1983) Das Phytoplankton des Süβwassers, Chlorophyceae (Grünalgen). Ordnung: Chlorococcales. E. Schweizerbartsche Verlagsbuchhandlung, Stuttgart, 1044 pp
Kowalska J, Wołowski K (2010) Pediastrum privum (Printz) Hegewald (Chlorophyceae) in Lake Małe Zmarłe, northern Poland. Oceanol Hydrobiol Stud 39(3):137–143. https://doi.org/10.2478/v10009-010-0034-4
Krammer K, Lange–Bertalot H (1988) Bacillariophyceae. 2. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Susswasserflora von Mitteleuropa, Band 2/2. Gustav Fisher Verlag, Jena, p 596
Krammer K, Lange–Bertalot H (1991) Bacillariophyceae. 3. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fisher Verlag, Stuttgart, p 576
Krasznai E, Borics G, Várbiró G, Abonyi A, Padisák J, Deák C, Tóthmérész B (2010) Characteristics of the pelagic phytoplankton in shallow oxbows. Hydrobiologia 639(1):173–184. https://doi.org/10.1007/s10750-009-0027-z
Mertens KN, Takano Y, Yamaguchi A, Gu H, Bogus K, Kremp A, Bagheri S, Matishov G, Matsuoka K (2015) The molecular characterization of the enigmatic dinoflagellate Kolkwitziella acuta reveals an affinity to the Excentrica section of the genus Protoperidinium. System Biodivers 13(6):509–524. https://doi.org/10.1080/14772000.2015.1078855
Moestrup Ø, Calado AJ (2018) Süßwasserflora von Mitteleuropa/Freshwater Flora of Central Europe VI: Dinophyceae. Springer Spektrum, Berlin, p 560
Molina N (2013) Conservation of rare or little-known species: biological, social, and economic considerations. Island Press, Washington, D.C., pp 389
Mosley LM (2015) Drought impacts on the water quality of freshwater systems; review and integration. Earth-Sci Rev 140:203–214. https://doi.org/10.1016/j.earscirev.2014.11.010
MSZ 12750–6:1971 (1971) Testing of surface waters. Determination of all solved and floating matter content. In Hungarian
MSZ 12750–20:1972 (1972) Water quality. Testing of surface waters. Determination of total nitrogen and organic nitrogen. In Hungarian
MSZ 448–15:1982 (1982) Drinking water analysis. Determination of chloride ion. In Hungarian
MSZ 448-11:1986 (1986) Drinking water analysis. Determination of basicity, hydrogen-carbonate ion, carbonate ion and hydroxil ion content. In Hungarian
MSZ 448–20:1990 (1990) Drinking water analysis. Determination of the chemical oxygen demand with potassium permanganate. In Hungarian
MSZ 1484–3:2006 (2006) Testing of waters. Part 3: Determination of dissolved, suspended and total metals in water by AAS and ICP–OES. In Hungarian
MSZ 1484–13:2009 (2009) Water quality. Part 13: Determination of nitrate and nitrite content by spectrophotometric method. In Hungarian
MSZ EN 1899–1:2000 (2000) Water quality – Determination of biochemical oxygen demand after n days (BODn). Part 1: Dilution and seeding method with allylthiourea addition (ISO 5815:1989, modified). In Hungarian
MSZ EN ISO 6878:2004 (2004) Water quality. Determination of phosphorus. Ammonium molybdate spectrometric method (ISO 6878:2004)
MSZ ISO 7150–1:1992 (1992) Water quality. Determination of ammonium. Part 1: Manual spectrophotometric method. In Hungarian
MSZ ISO 10260:1993 (1993) Water quality. Measurement of biochemical parameters. Spectrometric determination of the chlorophyll–a concentration. In Hungarian
Murdoch PS, Baron JS, Miller TL (2000) Potential effects of climate change on surface-water quality in North America. J Am Water Resour As 36:347–366. https://doi.org/10.1111/j.1752-1688.2000.tb04273.x
Németh J (1997) A guide for the identification of Euglenophyta occurring in Hungary, II., – KGI, Budapest. In Hungarian, pp 253
Németh J (2005) Red list of algae in Hungary. Acta Bot Hung 47:379–417. https://doi.org/10.1556/ABot.47.2005.3-4.7
OECD (1982) Eutrophicaton of Waters. Monitoring, assessment and control. Final report, OECD cooperative programme on monitoring of inland waters (Eutrophication control), Environment Directorate. Paris
Padisak J (1984) The algal flora and phytoplankton biomass of the Hungarian part of Lake Fertô II.: Southern open bays. BFB-Bericht 68:145–157
Padisák J (1993) The influence of different disturbance frequencies on the species richness, diversity and equitability of phytoplankton in shallow lakes. Hydrobiologia 249:135–156. https://doi.org/10.1007/BF00008850
Padisák J, Borics G, Fehér G, Grigorszky I, Oldal I, Schmidt A, Zámbóné–Doma Z (2003) Dominant species, functional assemblages and frequency of equilibrium phases in late summer phytoplankton assemblages in Hungarian small shallow lakes. Hydrobiologia 502:157–168. https://doi.org/10.1023/B:HYDR.0000004278.10887.40
Palik P (1940) A hazai tőzeglápok algái. II. A tólaki tőzeges láp Pomáz mellett. Index Horti Bot Univ Budapest 4:17–38
Palik P (1957) Studien über Hildenbrandtia rivularis (Liebm.) J. Ag. Ann Univ Sci Budap Rolando Eötvös Nominantae 1:205–218
Palik P (1966) Algae from the cave of Mátyás mount, Budapest, Hungary. Int J Speleol 2:155–164
Ponyi J, Tamas G (1964) Napszakos váItozások vizsgálata a tihanyi Belső-tó fito-és zooplanktonján. Állattani Közl 51:105–124
Reynolds CS (2006) The ecology of phytoplankton. Cambridge University Press, Cambridge, p 535. https://doi.org/10.1017/CBO9780511542145
Scheffer M (2004) Ecology of shallow lakes. Kluwer Academic Publishers, Dordrecht, Boston, London, p 357
Schmidt A (1975) Újabb adatok a Szelidi-tó limnologiai viszonyaihoz. Hidrol Közl 55:178–182
Schmidt A (1994) Main characteristics of the phytoplankton of the Southern Hungarian section of the River Danube. Hydrobiologia 289:97–108. https://doi.org/10.1007/BF00007412
Schmidt A, Fehér G (1998) A guide for the identification of Chlorococcales; Taxa occurring in Hungary, 1. KGI, Budapest. In Hungarian, pp 200
Schmidt A, Fehér G (1999) A guide for the identification of Chlorococcales; Taxa occurring in Hungary, 2. KGI, Budapest. In Hungarian, pp 203–378
Schmidt A, Fehér G (2001) A guide for the identification of Xanthophyceae (except Vaucheriales); Taxa occurring in Hungary. KGI, Budapest. In Hungarian, pp 217
Schmidt A, Vízkelety É, Mátyás K, Kiss KT (1991) Occurrence of Dicloster acutus Jao, Wei et Hu (Chlorococcales) in Hungary. Bot Közl 78:55–65. In Hunagrian
Schmidt A, Fehér G, Padisák J (2003) Some rare green algae occurring in the Danube river and its dead–and side–branches in southern Hungary. Biol Bratisl 58:475–482
Stenger–Kovács C, Hajnal É, Lengyel E, Buczkó K, Padisák J (2016) A test of traditional diversity measures and taxonomic distinctness indices on the benthic diatoms of soda pans in the Carpathian basin. Ecol Indic 64:1–8. https://doi.org/10.1016/j.ecolind.2015.12.018
Stenger–Kovács Cs, Lengyel E (2015) Taxonomical and distribution guide of diatoms in soda pans of Central Europe. Studia Bot Hung 46:3–203
Szabados M (1938) Flagellaten–Vegetation der „Holt–Tisza” bei Szentmihálytelek I. Bot Közl 36:107–119. In Hungarian
Szabó K, Kiss KT, Ector L, Kecskés M, Ács É (2004) Benthic diatom flora in a small Hungarian tributary of River Danube (Rákos-stream). Algol Stud 111:79–94. https://doi.org/10.1127/1864-1318/2004/0111-0079
Szemes G (1959) Systematische und floristisch-ökologische Bearbeitung von Bacillariophyceen des Szelider Sees. In E. Donaszy (ed.): Das Leber der Szelider Sees. Akademiai Kiado, Budapest 301–360
Szemes G (1969) The phytoplankton of the Hungarian reach of the Danube during the winter months. Ann Univ Sci Budapest Rolando Eotvos Nominatae 11:75–117
Tamás G (1949) Data for the knowledge of algae of the River Danube in Budapest section. Hidrol Közl 29:206–211. In Hunagrian
T–Krasznai E, B–Béres V, Buczkó K (2013) Adatok néhány ritka alga hazai előfordulásához. Kitaibelia 18:169–175
Uherkovich G (1959) Characteristics of the potamophytoplankton in the upper reach of the River Tisza at times of extremely high and extremely low water. Acta Biol Hung 3:21–22
Uherkovich G (1962) Adatok a zsombói erdő lápjainak mikrovegetációjához. Bot Közl 49:238–245. In Hungarian
Uherkovich G (1970) Beiträge zur Kenntnis der Algenvegetation der Natron (Szik-) Gewässer Ungarns. III. DasPhytoseston der Natronteiche bei Kunfehértó. Acta Bot Acad Sci Hung 16:405–426
Varga VI (1956) Adatok a szegedi Fehértó növényi mikrovegetációjához. Acta Acad Paed Szeged 1:169–179 (In Hungarian)
Vízkelety É (1987) The algological survey on Fekete–tó and Ördög–tó at Őrség. Praenorica 2:59–62
Wołowski K, Hindák F (2004) Taxonomic and ultrastructural studies of Trachelomonas Ehrenberg emend. Deflandre (Euglenophyta) from Slovakia. Nova Hedwigia 78(1–2):179–207. https://doi.org/10.1127/0029-5035/2004/0078-0179
Wołowski K, Hindák F (2005) Atlas of euglenophytes. Veda, Vijayawada, pp 136
Wołowski K, Walne PL (2007) Strombomonas and Trachelomonas species (Euglenophyta) from south-eastern USA. Eur J Phycol 42:4:409–431. https://doi.org/10.1080/09670260701702508
Acknowledgements
The authors are thankful to the Managing Editor, Katarína Hegedüšová and two anonymous reviewers for their valuable improvements to the manuscript draft.
Funding
Open access funding provided by ELKH Centre for Ecological Research. Authors are financially supported by the NKFIH FK 132 142 grant (VBB, ETK), by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO-00458-20-8 (VBB) and by the ÚNKP-20-5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (VBB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Enikő T-Krasznai. The first draft of the manuscript was written by Enikő T-Krasznai and both authors commented on previous versions of the manuscript. Viktoria B-Béres read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work. All data and material can be available from the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Table 1
Summary of data set for the chemical and physical parameters in the studied lakes and ponds: water temperature (ºC), Secchi-depth (cm), dissolved oxygen (DO–mg L− 1), oxygen saturation (OS–%), Chl-a (µg L− 1), pH, conductivity (COND–µS cm− 1), chemical oxygen demand (CODPs–mg L− 1), biological oxygen demand (BOD5–mg L− 1), hydrogencarbonate (HCO3 − 1–mg L− 1), chloride (Cl−–mg L− 1), soluble reactive phosphorus (SRP–mg L− 1), total amount of P-forms (TP–mg L− 1), ammonium (NH4+–mg L− 1), nitrite (NO2−–mg L− 1), nitrate (NO3−–mg L− 1), organic nitrogen (Organic N–mg L− 1), total amount of N-forms (TN–mg L− 1), total suspended solids (TSS–mg L− 1), and soluble reactive silica (Si–µg L− 1). (DOCX 37.7 KB)
Supplementary Table 2
Taxa rarity scale in Hungary and worldwide distribution of the 20 taxa rare in Hungary. (DOCX 38.5 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
T-Krasznai, E., B-Béres, V. Rarely mentioned species in Hungary: Can we step into the same lake?. Biologia 76, 1661–1673 (2021). https://doi.org/10.1007/s11756-021-00750-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00750-9