Abstract
Objective
The effect of one-inflow and two-inflow coronary surgical revascularization techniques inclosing skeletonized double mammary artery (BIMA) as T-graft on outcome is studied.
Methods
Early ad mid-term outcome of complete BIMA revascularization (C-T-BIMA) versus left-sided BIMA with right-sided aorto-coronary bypass (L-T-BIMA + R-CABG) is quantified and analyzed by multivariate logistic regression, Cox-regression, and Kaplan–Meier analysis in a series of 204 consecutive patients treated for triple-vessel coronary disease (3v-CAD).
Results
The L-T-BIMA + R-CABG technique (n = 104) enables higher number of total (4.02 ± 0.87 vs. 3.71 ± 0.69, p = 0.015) and right-sided (1.21 ± 0.43 vs. 1.02 ± 0.32, p = 0.001) coronary anastomoses, improves total bypass flow (125.88 ± 92.41 vs. 82.50 ± 49.26 ml, p < 0.0001) and bypass flow/anastomosis (31.83 ± 23.9 vs.22.77 ± 14.23, p = 0.001), and enhances completeness of revascularization (84% vs.69%, p = 0.014) compared to C-T-BIMA strategy (n = 100), respectively.
Although the incidence of MACCE was comparable in the two groups (8% vs.1.2%, p = 0.055), the progression of functional mitral regurgitation (FMR) was significantly lower after L-T-BIMA + R-CABG, then after C-T-BIMA (47% vs.64%, p = 0.017).
The use of C-T-BIMA-technique (HR = 4.2, p = 0.01) and preoperative RCA occlusion (HR = 3.006, p = 0.023) predicted FMR progression, whereas L-T-Graft + R-CABG technique protected against it (X2 = 14.04, p < 0.0001) independent of the anatomic and clinical complexity (Syntax score I: HR = 16.2, p = 0.156, Syntax score II: HR = 1.901, p = 0.751), of early- (0.96% vs.2%, p = 0.617) and mid-term mortality (5.8% vs.4%, p = 0.748) when compared to C-T-BIMA, respectively.
Conclusions
The two-inflow coronary revascularization by L-T-BIMA + R-CABG better protects against FMR progression without increasing MACCE and mortality. Older patients with RCA occlusion and reduced LV-EF benefit most from the two-inflow L-T-BIMA + R-CABG technique. Younger 3v-CAD patients with normal LV-EF can preferentially be managed with the one-inflow C-T-BIMA; however, long-term outcome remains to be revealed.
Similar content being viewed by others
Introduction
Triple-vessel coronary artery disease (3v-CAD) is more successfully treated by surgical revascularization than by catheter intervention [1] and revascularization to all three major vascular regions is an independent predictor of improved short- and long-term survival after CABG [2,3,4]. In situ left internal mammary artery (LIMA) grafting to the left anterior descending coronary artery (LAD) [5] and obtaining two conduits from a single sternotomy [6, 7] result in an additional survival benefit of bilateral IMA (BIMA) [8], especially by grafting the right IMA (RIMA) as T-graft to the left coronary system [9,10,11].
However, RIMA’s length might be insufficient to target all inferior coronary vessels [12, 13]. A single IMA inflow might also be insufficient to achieve maximal myocardial perfusion [14] and using only a T-graft has the potential of losing bypass flow because of the competitive flow of the native circulation [11, 13].
Ischemic cardiomyopathy and myocardial infarct size [15] can subsequently affect the geometry of the mitral valve apparatus, causing functional mitral regurgitation (FMR). Both left ventricular dysfunction and mitral annular dilatation were shown to be associated with FMR progression after CABG [16]. Even a mild degree of FMR portends substantial risk of cardiovascular mortality after acute myocardial infarction [17].
The present study aims to determine the influence of two different coronary surgical revascularization techniques featuring skeletonized BIMA as T-graft (Fig. 1) on the postoperative outcome including atrioventricular valve function.
Methods
This is a retrospective study on 3v-CAD patients treated with CABG performed by the same surgeons between January 1, 2016 and December 30, 2019.
Ethical statement
The study was approved by the institutional review boards at the Philipps University of Marburg, including a waiver of informed consent (ek_mr_110221_Wensauer-2).
Study population
We reviewed 204 consecutive patients who underwent first time CABG for 3v-CAD with BIMA T-Graft as sequential grafting (C-T-BIMA) or with BIMA as T-Graft for the revascularization of the left-sided coronary arteries and an aorto-coronary graft for the right-sided vessels (L–T-BIMA + R-CABG, Fig. 1).
Exclusion criteria were: previous cardiac surgeries, concomitant carotid surgery, concomitant ablation for atrial fibrillation with or without left atrial auricular closure, higher degree (3rd grade) of mitral valve regurgitation (FMR) or tricuspid valve regurgitation (TR) preoperatively, concomitant valve or aortic surgery, and grafting with other BIMA configurations.
Four experienced consultant surgeons performed the procedures. Each surgeons applied both surgical techniques. The selection of the type of technique was at surgeon’s choice and was guided by patient characteristics. In emergency cases with life-threatening hemodynamical instability, in situ LIMA-LAD bypass was always performed; however, a shorter RIMA was harvested in order to reduce the duration of hemodynamic instability or/and CPB-time. As a result, these patients always required an additional graft, finally receiving L–T-BIMA + R-CABG revascularization. On the other hand, elective diabetic patients, who previously underwent venous stripping and were having inadequate diameter of the radial artery in ultrasonography, always received C-T-BIMA revascularization.
Operative characteristics
All operations were performed under general anesthesia, via full sternotomy, using normothermic CPB and achieving cardiac arrest using either warm blood or cold crystalloid cardioplegia. All IMA grafts were harvested in skeletonized cold fashion, using clipping and minimal use of low-energy electrocautery. The RIMA was divided at its origin and connected end to side to the LITA as a T-graft. The in situ LIMA was always connected to the LAD. The radial arteries and the saphenous veins were harvested using an open surgical technique.
Data collection
Relevant history and preoperative and postoperative study variables were obtained from the clinical records.
The MediStim Butterfly Model BF 2004 transit time flow meter (MediStim AS, Oslo, Norway) was used for direct intra-operative assessment of every graft after weaning from CPB.
Intraoperative bypass flow assessment was performed in a similar clinical setting in all patients: after weaning from CPB, under DDD-pacing at 80 beats/min, mean blood pressure of 50–55 mmHg, and hemoglobin above 8 dg/ml.
Echocardiographic assessment was obtained according to the current guidelines [18]. Cardiac function was determined by transthoracic echocardiography at hospital arrival and at hospital discharge. Transesophageal echocardiography was performed intraoperatively in all patients, monitoring cardiac unloading during CPB and determining myocardial and valve function before CPB and after weaning from CPB.
After hospital discharge, patients were receiving cardiological surveillance with transthoracic echocardiography yearly.
Pharmaceutical treatment including ß-blockers, ACE inhibitors, diuretics, and statins was applied in all patients in conformity with the current recommendations of the European Society of Cardiology on ischemic cardiac disease, arterial hypertension and functional mitral regurgitation. Medication was adjusted in all patients during follow-up based on the clinical, laboratory, and echocardiographic findings.
Risk scores and definitions
Euroscore II and STS-score, Syntax score I (anatomical complexity), and Syntax score II for CABG (anatomical complexity, demographic, and clinical factors) were calculated as described elsewhere [19, 20]. Patients were subdivided by SS-value: group I (low or intermediate SS of ≤ 32), group II (high SS of > 32) for further analysis.
Revascularization was declared complete when the number of stenotic vessels (main coronaries or branch arteries) equaled the number of anastomoses [21].
The progression of FMR is defined as a relevant increase of the regurgitation volume caused by annular dilatation (Carpentier Type I) and/or left ventricular dilatation (Carpentier Type IIIb) without signs of degenerative disease and without new-onset of valve leaflet pathology.
Major adverse cardiac and cerebrovascular events (MACCE) were defined as death from any cause, nonfatal myocardial infarction, nonfatal stroke, and need for repeat revascularization.
Outcome measures
Primary outcome measure is a comparison of the surgical results (bypass flow measurements and completeness of revascularization) and of clinical outcome at hospital discharge (complications, MACCE and all-cause mortality) of the two techniques.
Secondary outcome measure is the determination of the postoperative evolution of FMR and TR at follow-up.
Tertiary outcome measure consisted of identification of predictors for postoperative MACCE and FMR progression.
Statistical analysis
Results are expressed as mean ± SD (standard deviation) for continuous variables and as percentages for categorical variables. Student's t-test, Kruskal–Wallis and Chi-Test evaluated differences between groups. Forward stepwise logistic regression and Cox regression were used to identify predictors for MACCE and FMR progression, respectively. Freedom from FMR progression was analyzed by Kaplan–Meier and compared by log rank test. A p value < 0.05 was considered significant. SPSS version 20.0 (IBM Corp Armonk, NY, USA) was used.
Results
Preoperative data
Baseline characteristics are listed in Table 1. Coronary angiography revealed more D1-Stenosis in C-T-BIMA patients (14% vs.6.7%, p = 0.044) and more RCA-Stenosis in L–T-BIMA + R-CABG patients (98% vs.84%, p = 0.003). Accordingly, less RCA-PCI was found in the L–T-BIMA + R-CABG group (4% vs. 14%, p = 0.013). Patients receiving L–T-BIMA + R-CABG suffered more from concomitant FMR and TR compared to C-T-BIMA (29.8% vs. 12%, p = 0.01, 14% vs.4%, p = 0.028, respectively). Risk scores were comparable between the groups.
Operative data and technical outcome
L-BIMA + R-CABG was more often performed urgently and required longer operation times (Table 2), resulting in longer intubation time and longer ICU stay (Table 3). More distal anastomoses, especially on the RCA system, were performed in the L-T-BIMA + R-CABG group leading to improved completeness of revascularization (84% vs. 69%, p = 0.014). Higher total bypass flows and increased flow/anastomosis were also achieved with L-T-BIMA + R-CABG (Table 2).
Clinical outcome
Early complications, MACCE, mortality at 30 days and at follow-up were similar between techniques (Table 3). LV function at follow-up improved in the L-BIMA + R-CABG group (57.22 ± 6.23 vs. 55.49 ± 5.48 preoperatively, p = 0.035). There was a maximum postoperative follow-up time of 5 years and minimum follow-up time of 24 month.
Although more patients in the L-T-BIMA + R-CABG group suffered from FMR preoperatively (30% vs. 12% p = 0.002), postoperative FMR progression was lower in the L-T-BIMA + R-CABG group (47% vs. 30%, p = 0.0152) than in C-T-BIMA (64% vs.12%, p < 0.0001).
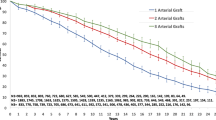
The Kaplan–Meier analysis revealed pronounced FMR progression in the C-T-BIMA group vs. L–T-BIMA + R-CABG (Fig. 2), independent of Syntax Scores (Fig. 3).
TR was higher in the L-T-BIMA + R-CABG than in C-T-BIMA (14% vs. 4%, p = 0.028) before the operation (Table 1), progressed postoperatively in the C-T-BIMA group (22% vs. 4%, p = 0.0002) and tended to decrease in the L-BIMA + R-CABG group (12% vs. 14%, p = 0.8943).
Risk factor analysis
No risk factors for MACCE were identified in our study (Supplemental Table 1).
Preoperative RCA occlusion (HR = 3.0, p = 0.023) and the type of operation (HR = 4.27, p = 0.010) influenced FMR progression in the entire cohort (Table 4), with RCA occlusion (HR = 9.932, p = 0.006), preoperative LV-EF (HR = 0.858, p = 0.019) and patient age (HR = 1.257, p = 0.012) predicted FMR progression in C-T-BIMA patient subgroup (Table 5, 6). P-value < 0.05 is statistically singificant.
Discussion
This study reveals that the adoption of the two-inflow L-T-BIMA + R-CABG technique in patients undergoing CABG for 3v-CAD is associated with improved completeness of revascularization when compared to the one-inflow C-T-BIMA grafting, without increasing the incidence of MACCE and mortality.
In addition, the L-T-BIMA + R-CABG impeded the progression of both FMR and TR more effectively than the C-T-BIMA revascularization technique.
Beyond expectations, in this cohort of largely uncomplicated CABG patients, total bypass flow increased with the number of the distal anastomoses, with the completeness of revascularization and with the number of inflows (Table 2). The Cox analysis (Table 4) disclosed a relative importance of bypass flow measurement, showing that neither the amount of bypass flow nor completeness of revascularization or the number of distal anastomoses were able to predict the progression of FMR after CABG. This discrepancy can be explained by the fact that the flow per each distal anastomosis was not measured but calculated in our study. Sequential bypass makes measurement between the distal coronary anastomosis difficult and does not allow direct quantification of the flow per anastomosis. Bypass flows change over time depending on the graft size, inflow, and peripheral resistance of the myocardial territory, with the later also changing after surgery, in direct proportion to the amount of the initially under-perfused myocardium that is recruited after revascularization.
Thus, the type of surgical technique and the presence of RCA occlusion proved to be the only predictors of FMR progression in 3v-CAD patients undergoing CABG (Tables 5, 6). In particular, patients with RCA occlusion benefit from the two-inflow revascularization. Presumably, the two-inflow L-T-BIMA + R-CABG technique, that is associated with a significantly higher number of distal anastomoses on both RCX and RCA coronary systems (Table 2), generates a significant augmentation of blood flow not only to the postero-inferior, but also to the left-lateral wall, thus improving the perfusion of both papillary muscles of the mitral valve. The multiple distal anastomoses on the terminal branches of the RCA system (PDA and RPL) additionally enhance the perfusion of the interventricular septum and posterior wall and thus improve left-side heart function, finally resulting in a relevant reduction of the tricuspid valve regurgitation.
These findings are in agreement with previous studies [6, 8, 9] revealing that competition of flow plays a crucial role in the long-term arterial grafting functionality, especially when sequential grafting is applied, and that the competitive flow occurring in sequential grafting is minimized when two inflows are applied. Thus, our results highlight that beyond completeness of revascularization, graft configuration is the most important factor influencing myocardial perfusion after CABG.
Thus, the hereby evaluated L-T-BIMA + R-CABG technique condenses the advantages of the fully arterial revascularization for the left-sided coronaries [6, 9] and offers a separate inflow for the revascularization of the right-sided coronary branches [14, 22, 23], resulting in outright myocardial perfusion.
First, these findings are in agreement with previous meta-analyses [24,25,26] demonstrating that BIMA grafting is associated with a significant reduction in early mortality, with improved long-term survival and with reduced risk of repeat revascularization compared to LIMA, without any adverse effects. In situ BIMA has been previously proved to provide greater bypass flow that in situ LIMA, especially when more than five branches were targeted [6, 9, 10], with the amount of bypass flow increasing proportionally with the number of distal anastomoses [5]. Moreover, there are both randomized [27, 28] and observational evidence [22, 23] demonstrating that composite grafting does not compromise graft patency or survival, probably because IMAs are the best-equipped arterial conduit to withstand the competition flow because of their endothelial function [25]. In situ LIMA also has the capacity to dilate to meet the local demands and even improves over time [26]. Furthermore, using the T-graft is not associated with a potential loss in the LIMA to LAD component of the graft [12, 13] and a patent LIMA to LAD affords significant benefit in terms of patient survival and freedom from MACCE [24,25,26, 29]. On other note, the use of BIMA as T-graft is associated with a reduced need for repeated revascularization [23, 26] and has been shown to provide an additional survival benefit and freedom from MACCE in patients undergoing CABG [14, 15].
Second, the use of a separate aortic inflow in the L-T-BIMA + R-CABG group is not a prognostic factor for MACCE. In agreement with previous studies [25, 28, 30], our data reveal that neither risk scores or type of operation, nor the number of distal anastomoses correlated with MACCE in CABG patients.
These findings may be linked to a well-conceived diagnostic and surgical strategy. Shortly, all our patients were scanned preoperatively for carotid artery stenosis. If stenosis was present, it was treated at the time of the CABG operation and these patients were excluded from the study. All patients suffering from atrial fibrillation received LAA closure with or without ablation at the time of CABG surgery, and atrial fibrillation was an exclusion criterion of our study. In addition, all patients over 70 years old and those with left main coronary artery disease received a thoracic CT scan for the preoperative evaluation of the aortic calcification. Intraoperative aortic cannulation was performed in Seldinger technique under TEE guidance. This overarching strategy could explain why the completion of an aortic inflow in the L-T-BIMA + R-CABG group did not increase the incidence of MACCE. Another reason is the preoperatively low risk profile of our cohort (Table 1). Scores that include clinical and angiographic variables, such as SS II, and global risk score such as Euroscore II and STS scores proved to be more suitable as predictors for MACCE than the purely angiographic SS I [23, 24]. In our study, there were no significant differences between the preoperative SS I and SS II of the two patient subgroups. The STS scores and Euroscore II were also similarly low (Table 1). Alike other studies [19, 20, 30], we found that risk scores, the type of operation, and the number of distal anastomoses did not correlate with MACCE in CABG patients (supplemental Table 1).
Notably, patients receiving L-T-BIMA + R-CABG showed a reduction in all-cause FMR progression at 5-year follow-up when compared to C-T-BIMA technique that was achieved with no increase in postoperative mortality or morbidity.
Whereas type of surgery and the preoperative RCA occlusion predicted FMR progression after CABG in the entire cohort (Table 4), LV-EF and RCA occlusion increased the risk of FMR progression only in the C-T-BIMA patients.
Comparison of freedom from progression of FMR in the two groups according to SS categories showed lower protection with C-T-BIMA technique in both—low–intermediate and high—SS I as well as SS II categories (Fig. 2). Thus, the L-T-BIMA + R-CABG technique performs better than C-T-BIMA in all aspects of postoperative outcome and independent of the SS scores.
In agreement with previous studies [19, 20, 30], our results also emphasize that SS scores alone cannot be utilized to guide the choice of surgical revascularization in patients with 3v-CAD.
Limitations
Although the techniques were equally used at both institutions by the same surgeons, techniques selection bias by surgeons may be present.
Differences in ventricular diameters and volumes, segmental dysfunctions, tenting heights and areas might have strengthened the study; however, they could not be extracted for all patients.
Statistical interrogation of a larger database might next confirm the present findings.
Conclusion
The L-T-BIMA + R-CABG revascularization technique that provides two separate inflows for the left- and right-sided grafting preserves all benefits of the BIMA T-graft revascularization without increasing the risk for MACCE, improves completeness of revascularization, and reduces the mid-term progression of FMR independent of the preoperative clinical and anatomical risks.
Older patients with reduced LV-EF, RCA occlusion, and higher Syntax-scores benefit most from the two-inflow L-T-BIMA + R-CABG technique.
Younger patients with 3v-CAD and normal LV-EF can preferentially be managed with the one-inflow C-T-BIMA; however, the long-term outcome remains to be revealed.
Data availability
The raw data that support the findings of this study are available from the corresponding author [T.B.A.], upon reasonable request.
Abbreviations
- 3v-CAD:
-
Three-vessel coronary artery disease
- CABG:
-
Coronary artery bypass grafting
- LIMA:
-
Left internal mammary artery
- BIMA:
-
Bilateral internal mammary artery
- LAD:
-
Left anterior descending coronary artery
- C-T-BIMA:
-
Complete revascularization with both internal mammary arteries as T-graft
- L-T-BIMA:
-
Left-sided revascularization with both internal mammary arteries as T-graft
- R-CABG:
-
Right-sided coronary artery bypass grafting with an aorto-coronary graft
- RCA:
-
Right coronary artery
- LV-EF:
-
Left ventricular ejection fraction
- CT-scan:
-
Computer tomographic scan
- TEE:
-
Transesophageal echocardiography
- MACCE:
-
Major adverse cardiac and cerebrovascular events
- FMR:
-
Mitral valve regurgitation
- TR:
-
Tricuspid valve regurgitation
- SS:
-
Syntax (synergy between PCI with taxus and cardiac surgery) Score
- Syntax:
-
Synergy between PCI with taxus and cardiac surgery
- MRI:
-
Magnetic resonance imaging
References
Deb S, Wijeysundera HC, Ko DT, et al. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013;310:2086–95.
Jones EL, Weintraub WS. The importance of completeness of revascularization during long-term follow-up after coronary artery operations. J Thorac Cardiovasc Surg. 1996;112:227–37.
Scott R, Blackstone EH, McCarthy PM, et al. Isolated bypass grafting of the left internal thoracic artery to the left anterior descending coronary artery: late consequences of incomplete revascularization. J Thorac Cardiovasc Surg. 2000;120:173–84.
Kozower BD, Moon MR, Barner HB, et al. Impact of complete revascularization on long-term survival after coronary artery bypass grafting in octogenarians. Ann Thorac Surg. 2005;80:112–6.
Nakajima H, Kobayashi J, Toda K, et al. Safety and efficacy of sequential and composite arterial grafting to more than five coronary branches in off-pump coronary revascularisation: assessment of intra-operative and angiographic bypass flow. Eur J Cardiothorac Surg. 2010;37:94–9.
Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005–12.
Endo M, Nishida H, Tomizawa Y, et al. Benefit of bilateral over single internal mammary artery grafts for multiple coronary artery bypass grafting. Circulation. 2001;104:2164–70.
Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularization on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–5.
Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg. 1999;117:855–72.
Grau JB, Ferrari G, Mak AW, et al. Propensity matched analysis of bilateral internal mammary artery versus single left internal mammary artery grafting at 17-year follow-up: validation of a contemporary surgical experience. Eur J Cardiothorac Surg. 2012;41:770–5.
Vallely MP, Edelman JJ, Wilson MK. Bilateral internal mammary arteries: evidence and technical considerations. Ann Cardiothorac Surg. 2013;2:570–7.
Kieser TM, Curran HJ, Rose MS, et al. Arterial grafts balance survival between incomplete and complete revascularization: a series of 1000 consecutive coronary artery bypass graft patients with 98% arterial grafts. J Thorac Cardiovasc Surg. 2014;147:75–83.
Vieira RD, Hueb W, Gersh BJ, et al. Effect of complete revascularization on 10-year survival of patients with stable multivessel coronary artery disease: MASS II trial. Circulation. 2012;126:S158–63.
Maniar HS, Barner HB, Bailey MS, et al. Radial artery patency: are aortocoronary conduits superior to composite grafting? Ann Thorac Surg. 2003;76:1498–503.
Cavalcante JL, Kusunose K, Obuchowski NA, et al. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2020;13:1489–501.
Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–53.
Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation. 1997;96:827–33.
Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:303–71.
Mohr FW, Rastan AJ, Serruys PW, et al. Complex coronary anatomy in coronary artery bypass graft surgery: impact of complex coronary anatomy in modern bypass surgery? Lessons learned from the SYNTAX trial after two years. J Thorac Cardiovasc Surg. 2011;141:130–40.
Cavalcante R, Sotomi Y, Mancone M, et al. Impact of the SYNTAX scores I and II in patients with diabetes and multivessel coronary disease: a pooled analysis of patient level data from the SYNTAX, PRECOMBAT, and BEST trials. Eur Heart J. 2017;38:1969–77.
Ong AT, Serruys PW. Complete revascularization: coronary artery bypass graft surgery versus percutaneous coronary intervention. Circulation. 2006;114:249–55.
Barner HB, Bailey M, Guthrie TJ, et al. Radial artery free and T graft patency as coronary artery bypass conduit over a 15-year period. Circulation. 2012;126:S140–4.
Lev-Ran O, Paz Y, Pevni D, et al. Bilateral internal thoracic artery grafting: midterm results of composite versus in situ crossover graft. Ann Thorac Surg. 2002;74:704–10.
Yi G, Shine B, Rehman SM, Altman DG, Taggart DP. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation. 2014;130:539–45.
Weiss AJ, Zhao S, Tian DH, et al. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg. 2013;2:390–400.
Takagi H, Goto SN, Watanabe T, et al. A meta-analysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;148:1282–90.
Nasso G, Coppola R, Bonifazi R, et al. Arterial revascularization in primary coronary artery bypass grafting: direct comparison of 4 strategies–results of the stand-in-Y mammary study. J Thorac Cardiovasc Surg. 2009;137:1093–100.
Muneretto C, Bisleri G, Negri A, et al. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: a prospective randomized comparison with conventional coronary artery bypass surgery. Circulation. 2003;108:29–33.
Iribarne A, Schmoker JD, Malenka DJ, et al. Does use of bilateral internal mammary artery grafting reduce long-term risk of repeat coronary revascularization? A multicenter analysis. Circulation. 2017;136:1676–85.
Lemesle G, Bonello L, de Labriolle A, et al. Prognostic value of the Syntax score in patients undergoing coronary artery bypass grafting for three-vessel coronary artery disease. Catheter Cardiovasc Interv. 2009;73:612–7.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and have no financial disclosures.
Institutional review board
The study was approved by the institutional review boards at the Philipps University of Marburg, that it conforms to the provisions of the Declaration of Helsinki (rev. Edinburgh 2000), including a waiver of informed consent (ek_mr_110221_Wensauer-2).
Informed consent
Written informed was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andrási, T.B., Glück, A.C., Talipov, I. et al. Sequential composite BIMA grafting for 3v-CAD: factors that predict successful outcome of the one-inflow and two-inflow revascularization techniques. Gen Thorac Cardiovasc Surg (2024). https://doi.org/10.1007/s11748-024-02022-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11748-024-02022-0