Abstract
Atrial-esophageal fistula is an extremely rare disease and a life-threatening complication after catheter ablation for atrial fibrillation. There is no consensus on the management or repair for atrial-esophageal fistula which has a high mortality rate. Here, we describe a lateral thoracotomy approach focused on simplifying the repair procedure for atrial-esophageal fistula in two patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catheter ablation for atrial fibrillation (AF) is widely utilized and considered safe (with 6% complications) [1]. Although atrial-esophageal fistula (AEF) is an uncommon disease, it is a life-threatening complication after catheter ablation for AF [2, 3]. The onset of AEF occurs several days to 2 months after catheter ablation. Its symptoms are diverse and include neurologic changes [2]. Because of its uncommonness, there is no gold standard for repairing AEF, which has a high mortality rate (up to 80%) [4]. Herein, we illustrate a lateral thoracotomy approach focusing on a simple repair for AEF in two patients.
Technique
Case 1
A 61 year-old man presented to the emergency department with a history of 3 days of fever. He had a history of radiofrequency catheter ablation of AF 1 month prior to admission, end-stage renal disease requiring hemodialysis, and pacemaker insertion. After admission to the hospital for evaluating the cause of fever, altered mental status was noted. Brain magnetic resonance imaging (MRI) showed a multifocal embolic infarction. Streptococcus salivarrius and Streptococcus mitis/oralis were found in blood culture. Chest computed tomography (CT) demonstrated AEF (Fig. 1a).
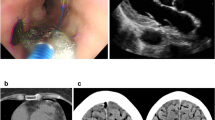
Perioperative findings about atrial-esophageal fistula in two patients. a A CT scan of the chest showing abnormal linear air densities in the left atrium demonstrating an AEF in case 1. b An endoscopic finding with a well-repaired previous fistula site in case 1. c A CT scan of the chest revealing air bubbles at the mediastinum suggestive of an AEF in case 2. d An endoscopic finding was unremarkable for a prior AEF site covered with a whitish exudate scar in case 2
A surgical strategy was urgently planned. After endotracheal general anesthesia, the patient was placed in the right lateral decubitus position. Single-lung ventilation was initiated. A thoracotomy was performed on the left 5th intercostal space. After left lung retraction, the pericardium was opened, revealing severe adhesions. Accessing the fistula through the pericardial space was attempted but was abandoned due to excessive adhesions. Attention was then turned to the outside of the pericardium. After retracting the left lung forward, we accessed below the left main bronchus and confirmed the left superior and inferior pulmonary veins. Then we approached behind the pulmonary veins and performed dissection. After opening the mediastinal pleura on the left hilum, the esophagus behind the pericardium was identified. Efforts were made to avoid any vascular damage because it is located more medially than the descending aorta. Between the esophagus and the posterior pericardial wall, the fistula site was observed (Fig. 2). We confirmed the 3 mm fistula with esophageal traction cautiously. Since there were no CT findings or surgical evidence suggesting an abscess, we judged that the situation was not severe in terms of localized infection. From posterior pericardial reflection, three pledgeted 4–0 prolene sutures were gently placed on the fistula on the pericardial side. Fistula ligation and resection were followed by an additional primary repair of the esophageal defect. The mucosal defect was repaired with interrupted 4–0 vicryl sutures, and the muscularis was reapproximated with interrupted 3–0 silk sutures. The repair procedure was finished after considerable irrigation of the chest and a chest tube was inserted.
Postoperatively, the patient was treated for sequelae from the initial stroke and pneumonia. After 1 week without oral nutrition, we confirmed a well-repaired fistula site without residual lesions on endoscopy (Fig. 1b). The patient was discharged on postoperative day 31 with a tracheal tube.
Case 2
A 70 year-old man underwent radiofrequency catheter ablation for AF. Fifty days following the procedure, he was admitted for cognitive decline. Multiple cerebral infarct lesions were observed on brain MRI. Blood cultures were taken to determine the cause of the patient’s fever. Multiple Streptococcus spp. were confirmed. A CT scan of the chest was suggestive of an AEF (Fig. 1c).
Surgery was urgently performed. The surgical approach was the same as in patient 1. After left thoracotomy on the 5th intercostal space, inspection of the pericardial space showed calcified adhesions. Through the posterior mediastinal space, the esophagus was exposed and pulled gently. The 3 mm fistula on the posterior aspect of the pericardium was isolated and carefully ligated with several pledgeted 4–0 prolene sutures. After fistulectomy, primary esophageal repair was performed in the same manner as the first case. After irrigation, a chest tube was then inserted. The wound was closed in a layered fashion.
The patient's postoperative program was focused on respiratory care with tracheostomy and rehabilitation for the cerebral infarction sequelae. Endoscopic findings were nonspecific after 1 week of fasting (Fig. 1d). The patient was discharged on postoperative day 23 with a tracheal tube.
Comments
Early diagnosis and surgical intervention are important in AEF. There are several treatment options for AEF, including esophageal stenting, intracardiac repair, extracardiac repair, and esophageal repair [2, 3, 5, 6].
Intracardiac repair of AEF requires sternotomy and cardiopulmonary bypass. A two-stage approach using intracardiac repair and esophageal repair has several disadvantages. It requires repositioning of the patient [7]. Another hybrid technique [8], including intracardiac repair and endoscopic clipping, has a risk for failure of the clipping procedure and the need for thoracic operation in cases of refisulization.
Patients with single-step repairs of AEF using a thoracotomy approach, with or without cardiopulmonary bypass, have also been reported [9, 10]. Our patients had a treatment strategy similar to the method reported by Khandhar et al. [10]. However, we did not use intercostal muscle flaps or any stapling devices. Unlike what was described by Khandhar and others, we focused on repairing the fistula in the simplest way possible.
The single-step lateral thoracotomy for AEF has several advantages in patients who are deemed to be free of left atrial active bleeding to the pericardial space. This lateral thoracotomy method can reduce operating time and eliminate the need for a cardiopulmonary bypass and a surgical repositioning. An AEF can be visually and reliably removed in one step. Also, due to the approach through the thoracic cavity, it has the advantage of effectively removing localized abscesses around the esophagus. Even if the problem on the left atrium side remains, there is room for future open heart surgery. Since stent insertion using endoscopy is not employed, the risk of air embolism associated with endoscopy can be eliminated. The lower esophagus runs toward the left hemithorax and passes behind the posterior wall of the left atrium, the left thoracotomy approach allows for better exposure of the lower esophagus. However, it is essential to exercise extreme caution during esophagus and fistula manipulation to prevent the occurrence of air embolism. Similar to our two cases who are less likely to have active bleeding of the left atrial wall, we can verify the intrathoracic findings first and close the fistula. Even in patients with multiple cerebral infarcts, it becomes burdensome to use cardiopulmonary bypass, since there is concern about cerebral hemorrhagic changes.
In conclusion, our experience suggests that one-step repair for AEF via lateral thoracotomy without cardiopulmonary bypass might be feasible in selected patients, especially in those with concomitant cerebral infarction.
Data availability
The data that support the findings of this study are available from the corresponding author, DYK, upon reasonable request.
References
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5.
Chavez P, Messerli FH, Casso Dominguez A, Aziz EF, Sichrovsky T, Garcia D, et al. Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart. 2015;2: e000257.
Singh SM, d’Avila A, Singh SK, Stelzer P, Saad EB, Skanes A, et al. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm. 2013;10:1591–7.
Ghia KK, Chugh A, Good E, Pelosi F, Jongnarangsin K, Bogun F, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009;24:33–6.
Hartman AR, Glassman L, Katz S, Chinitz L, Ross W. Surgical repair of a left atrial-esophageal fistula after radiofrequency catheter ablation for atrial fibrillation. Ann Thorac Surg. 2012;94:e91–3.
St Julien J, Putnam JB Jr, Nesbitt JC, Lambright ES, Petracek MR, Grogan EL. Successful treatment of atrioesophageal fistula by cervical esophageal ligation and decompression. Ann Thorac Surg. 2011;91:e85–6.
Guenthart BA, Sun B, De Biasi A, Fischbein MP, Liou DZ. Surgical technique for atrial-esophageal fistula repair after catheter ablation: an underrecognized complication. JTCVS Tech. 2020;4:169–72.
Kronenberger R, Mana F, Chierchia GB, La Meir M. A novel hybrid technique for the repair of an atrioesophageal fistula post-atrial radiofrequency ablation. Eur J Cardiothorac Surg. 2022;61:958–9.
Duda A, Beers K, Mitiek M. Surgical management of atrialesophageal fistula as a complication of atrial fibrillation ablation. J Surg Case Rep. 2022;2022:rjab497.
Khandhar S, Nitzschke S, Ad N. Left atrioesophageal fistula following catheter ablation for atrial fibrillation: off-bypass, primary repair using an extrapericardial approach. J Thorac Cardiovasc Surg. 2010;139:507–9.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JL and DYK participated in the study design, research, and manuscript writing. JY, SBH, YHK, and HWK were involved in research and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Yoon, J., Hong, S.B. et al. One-step thoracotomy approach for atrial-esophageal fistula repair without cardiopulmonary bypass. Gen Thorac Cardiovasc Surg 71, 681–684 (2023). https://doi.org/10.1007/s11748-023-01952-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-023-01952-5