Abstract
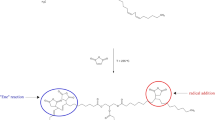
A fatty methyl ester product has been made using two routes. Soybean oil was thermally polymerized anaerobically without a catalyst at 330 °C and the material was then transesterified using base catalyst and methanol. Alternatively, a similar product can be obtained by heating methyl linoleate to the same temperature in a pressure reactor. The product structure was studied by NMR spectroscopy, gel permeation chromatography and mass spectrometry. It is a dimeric product which does not contain substituted cyclohexane structures. This evidence shows that the Diels–Alder reaction has not occurred under these conditions. This is in contradiction to many literature reports, but in agreement with a neglected paper from 1971. This correction has implications in both biodiesel and industrial oil products.
Graphical Abstract







Similar content being viewed by others
References
Gast LE, Schneider WJ, Forest CA, Cowan JC (1963) Composition of methyl esters from heat-bodied linseed oils. J Am Oil Chem Soc 40:287–289
Erhan SZ, Bagby MO (1998) Vegetable oil-based offset printing inks. US Patent 5713990
Erhan SZ, Bagby MO (1992) Vegetable oil-based printing ink. US Patent 5122188
Brod JS, France WG, Evans WL (1939) Thermal polymerization of ethyl eleostearate and 9,11- and 9,12-ethyl linoleate. Ind Eng Chem 31:114–118
Bradley TF, Johnston WB (1940) Drying oils and resins reactions involving the carbon-to-carbon unsaturation during the thermal treatment of some esters of unsaturated c18 fatty acids. Ind Eng Chem 32:802–809
Wang C, Erhan S (1999) Studies of thermal polymerization of vegetable oils with a differential scanning calorimeter. J Am Oil Chem Soc 76:1211–1216
Doll KM (2009) A convenient low-resolution NMR method for the determination of the molecular weight of soybean oil-based polymers. J Assoc Lab Autom 14:185–189
Henne AL, Turk A (1942) Conjugated diolefins by double bond displacement. J Am Chem Soc 64:826–828
Cowan JC (1949) lsomerization reactions of drying oils. Ind Eng Chem 41:294–304
Clingman AL, Sutton DA (1953) The chemistry of polymerized oils. II. Dehydro-polymers of methyl linoleate and methyl stearate. J Am Oil Chem Soc 30:53–56
Larock RC, Dong X, Chung S, Kishan Reddy C, Ehlers LE (2001) Preparation of conjugated soybean oil and other natural oils and fatty acids by homogeneous transition metal catalysis. J Am Oil Chem Soc 78:447–453
Paschke RF, Peterson LE, Wheeler DH (1964) Dimer acid structures. The thermal dimer of methyl 10-trans, 12-trans, linoleate. J Am Oil Chem Soc 41:723–727
Paschke RF, Jackson JE, Wheeler DH (1952) Thermal polymerization of drying oils isomers of methyl linoleate. Ind Eng Chem 44:1113–1118
Wheeler DH, Milun A, Linn F (1970) Dimer acid structures: Cyclic structures of clay catalyzed dimers of normal linoleic acid, 9-cis, 12-cis-octadecadienoic acid. J Am Oil Chem Soc 47:242–244
Beal RE, Lauderback LL, Ford JR (1975) Soybean soapstock utilization: fatty acid adducts with ethylene and 1-butene. J Am Oil Chem Soc 52:400–403
Eren T, Kusefoglu SH, Wool R (2003) Polymerization of maleic anhydride-modified plant oils with polyols. J Appl Polym Sci 90:197–202
Biermann U, Butte W, Eren T, Haase D, Metzger JO (2007) Regio- and stereoselective Diels-Alder additions of maleic anhydride to conjugated triene fatty acid methyl esters. Eur J Org Chem 2007:3859–3862
Figge K (1971) Dimeric fatty acid [1–14C] methyl esters. I. Mechanisms and products of thermal and oxidative-thermal reactions of unsaturated fatty acid esters—literature review. Chem Phys Lipids 6:159–177
Gertz C, Klostermann S, Kochhar SP (2000) Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Acknowledgments
Karl E. Vermillion is acknowledged for NMR analysis and discussion. This research was part of a joint effort by the Agricultural Research Service of the United States Department of Agriculture, Oil Products Group, Peoria, IL and the Tribology Group, Chemical Engineering Department of the Pennsylvania State University, University Park, PA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
About this article
Cite this article
Arca, M., Sharma, B.K., Price, N.P.J. et al. Evidence Contrary to the Accepted Diels–Alder Mechanism in the Thermal Modification of Vegetable Oil. J Am Oil Chem Soc 89, 987–994 (2012). https://doi.org/10.1007/s11746-011-2002-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-2002-x