Abstract
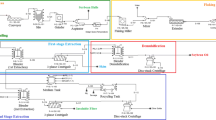
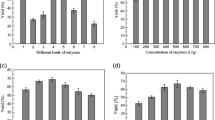
An aqueous enzymatic procedure for oleosome fractionation from 25 g of soy flour was developed in our laboratory. This fractionation procedure was evaluated with 75 kg using pilot plant equipment to evaluate the effect of the scale-up on the recovery, proximate composition, soybean storage protein profiles, and subcellular microstructure of oleosome fractions. The process included enzymatic hydrolysis, grinding, and centrifugation, respectively. Pilot-scale grinding and centrifugation of the slurry were accomplished with a Stephan® Microcut mill grinder and a three phase decanter. A blender and swinging bucket rotor were used for the laboratory-scale fractionation. The oleosome fractions recovered in the pilot plant were similar in oil and protein content to those obtained in the laboratory. The pilot-scale process resulted in a significantly higher oil yield of 93.40% as total oleosomes compared to that of 76.83% achieved in the laboratory. Urea–SDS gel electrophoresis of proteins extracted from the oleosomes and supernatant from the pilot-scale fractionation had similar profiles to those obtained in the laboratory. Electron microscopy verified that the structure of isolated oleosomes was virtually identical with that of in situ oleosomes. This work confirms that large-scale fractionation of oleosomes from full fat soybean flour can be accomplished.



Similar content being viewed by others
References
Bair CW, Snyder HE (1980) Electron microscopy of soybean lipid bodies. J Am Oil Chem Soc 57:279–282
Murphy DJ (1993) Structure, function and biogenesis of storage lipid bodies and oleosins in plants. Prog Lipid Res 32:247–280
Huang AHC (1994) Structure of plant seed oil bodies. Curr Opin Struct Biol 4:493–498
Herman EM (1995) Cell and molecular biology of seed oil bodies. In: Kigel Galili G, Dekker M (eds) Seed development and germination, New York, pp 195–214
Napier JA, Stobart AK, Shewry PR (1996) The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol Biol 31:945–956
Tzen JTC, Huang AHC (1992) Surface structure and properties of plant seed oil bodies. Cell Biol 117:327–335
Andrea A, Jolivet P, Boulard C, Larre C, Froissard M, Chardot T (2007) Selective one-step extraction of Arabidopsis thaliana seed oleosins using organic solvents. J Agric Food Chem 55:10008–10015
Yatsu LY, Jacks TJ (1972) Spherosome membranes: half unit membranes. Plant Physiol 49:937–943
Huang AHC (1992) Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol 177–200
Beisson F, Ferte N, Bruley S, Voultoury R, Verger R, Arondel V (2001) Oil-bodies as substrates for lipolytic enzymes. Biochim Biophys Acta 1531:47–58
Iwanaga D, Gray DA, Fisk ID, Decker EA, Weiss J, McClements DJ (2007) Extraction and characterization of oil bodies from soybeans: a natural source of pre-emulsified soybean oil. J Agric Food Chem 55:8711–8716
Iwanaga D, Gray DA, Decker EA, Weiss J, McClements DJ (2008) Stabilization of soy oil bodies using protective pectin coating formed by electrostatic deposition. J Agric Food Chem 56:2240–2245
Jacks TJ, Yatsu LY, Altschul AM (1967) Isolation and characterization of peanut spherosomes. Plant Physiol 42:585–597
Tzen JTC, Peng CC, Cheng DJ, Chen ECF, Chui JMH (1997) A new method for seed oil body purification and examination of oil body integrity following germination. J Biochem 121:762–768
Millichip M, Tatham AS, Jackson F, Griffiths G, Shewry PR, Stobart AK (1996) Purification and characterization of oil-bodies (oleosomes) and oil-body boundary proteins (oleosins) from the developing cotyledons of sunflower (Helianthus annuus L.). J Biochem 314:333–337
Nikifordis C, Kiosseoglou V (2009) Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil in water emulsion. J Agric Food Chem 57:5591–5596
White DA, Fisk ID, Makkhun S, Gray DA (2009) In vitro assessment of the bioaccessibility of tocopherol and fatty acids from sunflower seed oil bodies. J Agric Food Chem 57:5720–5726
Chen Y, Ono T (2010) Simple extraction method of non-allergenic intact soybean oil bodies that are thermally stable in an aqueous medium. J Agric Food Chem 58:7402–7407
Kapchie VN, Wei D, Hauck C, Murphy PA (2008) Enzyme-assisted aqueous extraction of oleosomes from soybeans (Glycine max). J Agric Food Chem 56:1766–1771
Towa TL, Kapchie VN, Hauck C, Murphy PA (2010) Enzyme-assisted aqueous extraction of oil from isolated oleosomes of soybean flour. J Am Oil Chem Soc 87:347–354
Chipley JR (1983) Sodium benzoate and benzoic acid. In: Davidson PM, Branen AL (eds) Antimicrobials in foods. Marcel Dekker, New York, pp 11–35
American Association of Cereal Chemists (AACC) (1983) Method 30–25, Approved methods of the American Association of Cereal Chemists, 8th edn. St. Paul, MN
Association of Official Analytical Chemists (AOAC) (1983) Official methods of analysis, 15th edn. AOAC, Washington, DC
Jung S, Rickert DA, Deak NA, Aldin ED, Recknor J, Johnson LA, Murphy PA (2003) Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J Am Oil Chem Soc 80:1169–1173
Sabir SM, Hayat I, Hussain HI, Gardezi SRA (2003) Proximate analysis of mushrooms of Azad Kashmir. Pak J Plant Pathol 2:97–101
Lamsal BP, Reitmeier C, Murphy PA, Johnson LA (2006) Enzymatic hydrolysis of extruded-expelled soy flour and resulting functional properties. J Am Oil Chem Soc 83:731–737
Wu S, Murphy PA, Johnson LA, Reuber MA, Fratzke AR (2000) Simplified process for soybean glycinin and β-conglycinin fractionation. J Agric Food Chem 48:2702–2708
Kapchie VN, Towa TL, Hauck C, Murphy PA (2010) Recycling of aqueous supernatants in soybean oleosome fractionation. J Am Oil Chem Soc 87:223–231
Jung S, Lamsal BP, Stepien V, Johnson LA, Murphy PA (2006) Functionality of soy proteins produced by enzyme-assisted extraction. J Am Oil Chem Soc 83:71–78
Chabrand RM, Kim HJ, Zhang C, Glatz CE, Jung S (2008) Destabilization of the emulsion formed during aqueous extraction of soybean oil. J Am Oil Chem Soc 85:383–390
Murphy DJ, Cummins L (1989) Seed oil-bodies isolation, composition and role of oil-body apolipoproteins. Phytochemistry 28:2063–2069
Jacks TJ, Hensarling TP, Neucere JN, Yatsu LY, Barker RH (1990) Isolation and physicochemical characterization of the half-unit membranes of oilseed lipid bodies. J Am Oil Chem Soc 67:353–361
Lamsal BP, Johnson LA (2007) Separating oil from aqueous extraction fractions of soybean. J Am Oil Chem Soc 84:785–792
Wu J, Johnson LA, Jung S (2009) Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soy bean flakes. Bioresour Technol 100:527–533
Acknowledgments
This work was supported by USDA Special Grant (20063443217128). Genencor, a Danisco company, is gratefully acknowledged for providing the enzymes.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Towa, L.T., Kapchie, V.N., Hauck, C. et al. Pilot Plant Recovery of Soybean Oleosome Fractions by an Enzyme-Assisted Aqueous Process. J Am Oil Chem Soc 88, 733–741 (2011). https://doi.org/10.1007/s11746-010-1716-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1716-5