Abstract
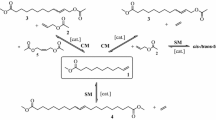
Studies were conducted in the synthetic conversion of oleic acid to mid-chain branched fatty acids. Methyl oleate was brominated in the allylic positions. Reaction of the allylic bromides with lithium dimethylcuprate gave primarily the desired branched-chain derivatives (93% of product mixture). The product had a significantly lower crystallization temperature in comparison with methyl oleate. Reaction of the allylic bromides with lithium di-n-butylcuprate or lithium di-sec-butylcuprate also gave branched-chain derivatives, but in this instance there was the complication of attack on the ester functionality in the fashion of a Grignard reagent. Details of the syntheses and the properties of the products (with emphasis on low-temperature properties) are discussed.







Similar content being viewed by others
References
Knothe G, Dunn RO, Bagby MO (1997) Biodiesel: the use of vegetable oils and their derivatives as alternative diesel fuels. In: ACS Symp. Ser. 666 (Fuels and Chemicals from Biomass). American Chemical Society, Washington, pp 172–208
Dunn RO (1999) Thermal analysis of alternative diesel fuels from vegetable oils. J Am Oil Chem Soc 76:109–115
Lee I, Johnson LA, Hammond EG (1995) Use of branched-Chain esters to reduce the crystallization temperature of biodiesel. Ibid 72:1155–1160
Foglia TA, Nelson LA, Dunn RO, Marmer WN (1997) Low-temperature properties of alkyl esters of tallow and grease. Ibid 74:951–955
Wu W-H, Foglia TA, Marmer WN, Dunn RO, Goering CE, Briggs TE (1998) Low-temperature property and engine performance evaluation of ethyl and isopropyl esters of tallow and grease. Ibid 75:1173–1178
Gunstone FD (1994) Fatty Acid Structure. In: Gunstone FD, Harwood JL, Padley FB (eds) The lipid handbook. Chapman & Hall, London, pp 11–12
Polgar N (1971) Natural alkyl-branched long-chain acids. Top lipid chem 2:207–246
Yang Z (2001) Anticancer effects of specific branched-chain fatty acids and related production process. US. Patent No. 6,214,875
Corey EJ, Posner GH (1967) Selective formation of carbon-carbon bonds between unlike groups using organocopper reagents. J Am Chem Soc 89:3911–3912
Still WC, Kahn M, Mitra A (1978) Rapid chromatographic techniques for preparative separation with moderate resolution. J Org Chem 43:2923–2925
Corey EJ, Posner GH (1968) Carbon-carbon bond formation by selective coupling of n-alkylcopper reagents with organic halides. J Am Chem Soc 90:5615–5616
van Tamelen EE, McCormick JP (1970) Synthesis of Cecropia Juvenile Hormone from trans, trans-Farnesol. Ibid 92:737–738
The Lipid Library (2006) 1H-NMR spectroscopy of fatty acids with non-conjugated double bonds. http://www.lipidlibrary.co.uk./nmr/1NMRdbs/index.htm
Posner GH, Whitten CE, McFarland PE (1972) Organocopper chemistry. Halo-, cyano-, and carbonyl-substituted ketones from the corresponding acyl chlorides and organocopper reagents. J Am Chem Soc 94:5106–5108
Dorman DE, Jautelat M, Roberts JD (1971) Carbon-13 nuclear magnetic resonance spectroscopy. Quantitative correlations of the carbon chemical shifts of acyclic alkenes. J Org Chem 36:2757–2766
Acknowledgments
Casey Grimm (SRRC) and Steven Lloyd (SRRC) conducted the GC/MS studies; Gary Strahan (USDA, ARS, ERRC, Wyndmoor, PA) conducted the NMR experiments; Navzer Sachinvala (SRRC) provided technical advice. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dailey, O.D., Prevost, N. Conversion of Methyl Oleate to Branched-Chain Derivatives. J Amer Oil Chem Soc 84, 565–571 (2007). https://doi.org/10.1007/s11746-007-1077-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1077-x